Spatial Relationships between Molecular Pathology and Neurodegeneration in the Alzheimer's Disease Continuum
- PMID: 32808011
- PMCID: PMC7727356
- DOI: 10.1093/cercor/bhaa184
Spatial Relationships between Molecular Pathology and Neurodegeneration in the Alzheimer's Disease Continuum
Abstract
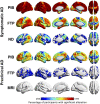
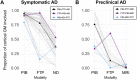
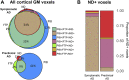
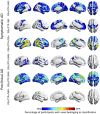
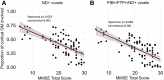
A deeper understanding of the spatial relationships of β-amyloid (Aβ), tau, and neurodegeneration in Alzheimer's disease (AD) could provide insight into pathogenesis and clinical trial design. We included 81 amyloid-positive patients (age 64.4 ± 9.5) diagnosed with AD dementia or mild cognitive impairment due to AD and available 11C-PiB (PIB), 18F-Flortaucipir (FTP),18F-FDG-PET, and 3T-MRI, and 31 amyloid-positive, cognitively normal participants (age 77.3 ± 6.5, no FDG-PET). W-score voxel-wise deviation maps were created and binarized for each imaging-modality (W > 1.64, P < 0.05) adjusting for age, sex, and total intracranial volume (sMRI-only) using amyloid-negative cognitively normal adults. For symptomatic patients, FDG-PET and atrophy W-maps were combined into neurodegeneration maps (ND). Aβ-pathology showed the greatest proportion of cortical gray matter suprathreshold voxels (spatial extent) for both symptomatic and asymptomatic participants (median 94-55%, respectively), followed by tau (79-11%) and neurodegeneration (41-3%). Amyloid > tau > neurodegeneration was the most frequent hierarchy for both groups (79-77%, respectively), followed by tau > amyloid > neurodegeneration (13-10%) and amyloid > neurodegeneration > tau (6-13%). For symptomatic participants, most abnormal voxels were PIB+/FTP+/ND- (median 35%), and the great majority of ND+ voxels (91%) colocalized with molecular pathology. Amyloid spatially exceeded tau and neurodegeneration, with individual heterogeneities. Molecular pathology and neurodegeneration showed a progressive overlap along AD course, indicating shared vulnerabilities or synergistic toxic mechanisms.
Keywords: amyloid; neurodegeneration; spatial extent; tau; topography.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC et al. . 2011. The diagnosis of mild cognitive impairment due to Alzheimer’s Disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s Disease. Alzheimers Dement. 7:270–279. - PMC - PubMed
-
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38:95–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

