Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-enhanced Raman Scattering
- PMID: 32808471
- PMCID: PMC7693192
- DOI: 10.1002/asia.202000847
Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-enhanced Raman Scattering
Abstract
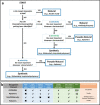
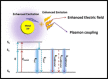
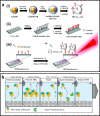
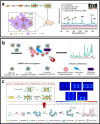
Metal nanoparticles (NP) that exhibit localized surface plasmon resonance play an important role in metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS). Among the optical biosensors, MEF and SERS stand out to be the most sensitive techniques to detect a wide range of analytes from ions, biomolecules to macromolecules and microorganisms. Particularly, anisotropic metal NPs with strongly enhanced electric field at their sharp corners/edges under a wide range of excitation wavelengths are highly suitable for developing the ultrasensitive plasmon-enhanced biosensors. In this review, we first highlight the reliable methods for the synthesis of anisotropic gold NPs and silver NPs in high yield, as well as their alloys and composites with good control of size and shape. It is followed by the discussion of different sensing mechanisms and recent advances in the MEF and SERS biosensor designs. This includes the review of surface functionalization, bioconjugation and (directed/self) assembly methods as well as the selection/screening of specific biorecognition elements such as aptamers or antibodies for the highly selective bio-detection. The right combinations of metal nanoparticles, biorecognition element and assay design will lead to the successful development of MEF and SERS biosensors targeting different analytes both in-vitro and in-vivo. Finally, the prospects and challenges of metal-enhanced biosensors for future nanomedicine in achieving ultrasensitive and fast medical diagnostics, high-throughput drug discovery as well as effective and reliable theranostic treatment are discussed.
Keywords: Assay design; Fluorescence detection; Metal nanoparticles; Plasmonic enhancement; SERS Biosensors.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- None
-
- Yu H., Peng Y., Yang Y., Li Z.-Y., npj Computat. Mater. 2019, 5, 45;
-
- Wang X., Huang S.-C., Hu S., Yan S., Ren B., Nat. Rev. Phys. 2020, 2, 253–271.
-
- None
-
- Tayebi M., Tavakkoli Yaraki M., Ahmadieh M., Tahriri M., Vashaee D., Tayebi L., Colloid Polym. Sci. 2016, 294, 1453–1462;
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

