Knockout of SORBS2 Protein Disrupts the Structural Integrity of Intercalated Disc and Manifests Features of Arrhythmogenic Cardiomyopathy
- PMID: 32808564
- PMCID: PMC7660791
- DOI: 10.1161/JAHA.119.017055
Knockout of SORBS2 Protein Disrupts the Structural Integrity of Intercalated Disc and Manifests Features of Arrhythmogenic Cardiomyopathy
Abstract
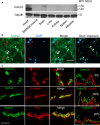
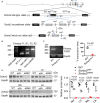
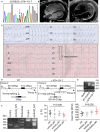
Background Sorbs2b (sorbin and SH3 domain-containing 2b) was recently identified as a cardiomyopathy gene from a zebrafish mutagenesis screen. However, cardiac functions of its mammalian ortholog remain elusive. Methods and Results We conducted a detailed expression and subcellular localization analysis of Sorbs2 ortholog in mice and a phenotypic characterization in Sorbs2 knockout mice. Sorbs2 is highly expressed in the mouse heart and encodes an adhesion junction/desmosome protein that is mainly localized to the intercalated disc. A mutation with near complete depletion of the Sorbs2 protein in mice results in phenotypes characteristic of human arrhythmogenic cardiomyopathy (ACM), including right ventricular dilation, right ventricular dysfunction, spontaneous ventricular tachycardia, and premature death. Sorbs2 is required to maintain the structural integrity of intercalated disc. Its absence resulted in profound cardiac electrical remodeling with impaired impulse conduction and action potential derangements. Targeted sequencing of human patients with ACM identified 2 rare splicing variants classified as likely pathogenic were in 2 unrelated individuals with ACM from a cohort of 59 patients with ACM. Conclusions The Sorbs2 knockout mouse manifests several key features reminiscent of human ACM. Although the candidacy of SORBS2 as a new ACM-susceptibility gene is supported by preliminary human genetics study, future validation in larger cohorts with ACM is needed.
Keywords: arrhythmogenic cardiomyopathy; intercalated disc; sorbin and SH3 domain‐containing 2; susceptibility gene.
Conflict of interest statement
None.
Figures



References
-
- Gandjbakhch E, Redheuil A, Pousset F, Charron P, Frank R. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:784–804. - PubMed
-
- Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. - PubMed
-
- Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. - PubMed
-
- Bennett RG, Haqqani HM, Berruezo A, Della Bella P, Marchlinski FE, Hsu CJ, Kumar S. Arrhythmogenic cardiomyopathy in 2018–2019: ARVC/ALVC or both? Heart Lung Circ. 2019;28:164–177. - PubMed
-
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet. 2000;355:2119–2124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

