APOE and dementia - resequencing and genotyping in 105,597 individuals
- PMID: 32808727
- PMCID: PMC7984319
- DOI: 10.1002/alz.12165
APOE and dementia - resequencing and genotyping in 105,597 individuals
Abstract
Introduction: The mechanism behind the strong association between the ɛ2/ɛ3/ɛ4 apolipoprotein E gene (APOE) polymorphism and Alzheimer's disease is not well-characterized. Because low plasma levels of apoE associate with risk of dementia, genetic variants altering apoE levels in general may also associate with dementia.
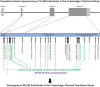
Methods: The APOE gene was sequenced in 10,369 individuals, and nine amino acid-changing variants with frequencies ≥2/10,000 were further genotyped in 95,228 individuals. Plasma apoE levels were measured directly.
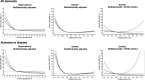
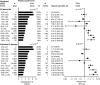
Results: Risk of all dementia and Alzheimer's disease (AD) increased with decreasing genetically determined apoE levels (P = 5 × 10-4 and P = 1 × 10-4 after APOE ɛ2/ɛ3/ɛ4 adjustment). Hazard ratios (95% confidence intervals) for all dementia and AD were 2.76 (1.39 to 5.47) and 4.92 (2.36 to 10.29) for the group with the genetically lowest apoE versus ɛ33.
Discussion: We found that genetically low apoE levels increase and genetically high levels decrease risk, beyond ɛ2/ɛ3/ɛ4. This underscores that dementia risk more likely relates to variants affecting levels of apoE.
Keywords: APOE; Alzheimer's disease; apolipoprotein E; dementia; genetics; rare variation.
© 2020 The Authors. Alzheimer's & Dementia published by Wiley Periodicals, Inc. on behalf of Alzheimer's Association.
Figures


References
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63‐75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

