The Virological, Immunological, and Imaging Approaches for COVID-19 Diagnosis and Research
- PMID: 32808850
- PMCID: PMC7435207
- DOI: 10.1177/2472630320950248
The Virological, Immunological, and Imaging Approaches for COVID-19 Diagnosis and Research
Abstract
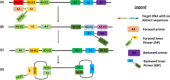
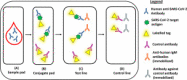
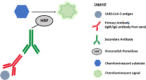
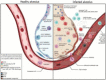
In 2019, a novel coronavirus (SARS-CoV-2) was found to cause a highly contagious disease characterized by pneumonia. The disease (COVID-19) quickly spread around the globe, escalating to a global pandemic. In this review, we discuss the virological, immunological, and imaging approaches harnessed for COVID-19 diagnosis and research. COVID-19 shares many clinical characteristics with other respiratory illnesses.Accurate and early detection of the infection is pivotal to controlling the outbreak, as this enables case identification, isolation, and contact tracing. We summarize the available literature on current laboratory and point-of-care diagnostics, highlight their strengths and limitations, and describe the emerging diagnostic approaches on the horizon.We also discuss the various research techniques that are being used to evaluate host immunity in laboratory-confirmed patients. Additionally, pathological imaging of tissue samples from affected patients has a critical role in guiding investigations on this disease. Conventional techniques, such as immunohistochemistry and immunofluorescence, have been frequently used to characterize the immune microenvironment in COVID-19. We also outline the emerging imaging techniques, such as the RNAscope, which might also aid in our understanding of the significance of COVID-19-specific biomarkers, such as the angiotensin-converting enzyme 2 (ACE2) cellular receptor.Overall, great progress has been made in COVID-19 research in a short period. Extensive, global collation of our current knowledge of SARS-CoV-2 will provide insights into novel treatment modalities, such as monoclonal antibodies, and support the development of a SARS-CoV-2 vaccine.
Keywords: COVID-19; diagnostics; immunology; pathology; specific T cells.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report—162. https://www.who.int/docs/default-source/coronaviruse/20200630-covid-19-s... (accessed Aug 8, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

