Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial
- PMID: 32809003
- PMCID: PMC7435341
- DOI: 10.1001/jama.2020.11946
Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial
Abstract
Importance: The combination of ascorbic acid, corticosteroids, and thiamine has been identified as a potential therapy for septic shock.
Objective: To determine whether the combination of ascorbic acid, corticosteroids, and thiamine attenuates organ injury in patients with septic shock.
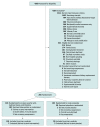
Design, setting, and participants: Randomized, blinded, multicenter clinical trial of ascorbic acid, corticosteroids, and thiamine vs placebo for adult patients with septic shock. Two hundred five patients were enrolled between February 9, 2018, and October 27, 2019, at 14 centers in the United States. Follow-up continued until November 26, 2019.
Interventions: Patients were randomly assigned to receive parenteral ascorbic acid (1500 mg), hydrocortisone (50 mg), and thiamine (100 mg) every 6 hours for 4 days (n = 103) or placebo in matching volumes at the same time points (n = 102).
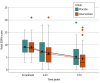
Main outcomes and measures: The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score (range, 0-24; 0 = best) between enrollment and 72 hours. Key secondary outcomes included kidney failure and 30-day mortality. Patients who received at least 1 dose of study drug were included in analyses.
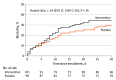
Results: Among 205 randomized patients (mean age, 68 [SD, 15] years; 90 [44%] women), 200 (98%) received at least 1 dose of study drug, completed the trial, and were included in the analyses (101 with intervention and 99 with placebo group). Overall, there was no statistically significant interaction between time and treatment group with regard to SOFA score over the 72 hours after enrollment (mean SOFA score change from 9.1 to 4.4 [-4.7] points with intervention vs 9.2 to 5.1 [-4.1] points with placebo; adjusted mean difference, -0.8; 95% CI, -1.7 to 0.2; P = .12 for interaction). There was no statistically significant difference in the incidence of kidney failure (31.7% with intervention vs 27.3% with placebo; adjusted risk difference, 0.03; 95% CI, -0.1 to 0.2; P = .58) or in 30-day mortality (34.7% vs 29.3%, respectively; hazard ratio, 1.3; 95% CI, 0.8-2.2; P = .26). The most common serious adverse events were hyperglycemia (12 patients with intervention and 7 patients with placebo), hypernatremia (11 and 7 patients, respectively), and new hospital-acquired infection (13 and 12 patients, respectively).
Conclusions and relevance: In patients with septic shock, the combination of ascorbic acid, corticosteroids, and thiamine, compared with placebo, did not result in a statistically significant reduction in SOFA score during the first 72 hours after enrollment. These data do not support routine use of this combination therapy for patients with septic shock.
Trial registration: ClinicalTrials.gov Identifier: NCT03389555.
Conflict of interest statement
Figures
Comment in
-
[Focus general intensive care medicine. Intensive care studies from 2020/2021].Anaesthesist. 2021 Oct;70(10):888-894. doi: 10.1007/s00101-021-00976-x. Epub 2021 Jul 29. Anaesthesist. 2021. PMID: 34324037 Free PMC article. German. No abstract available.

