Drug-Drug Interactions between Direct Oral Anticoagulants and Hepatitis C Direct-Acting Antiviral Agents: Looking for Evidence Through a Systematic Review
- PMID: 32809123
- PMCID: PMC7595962
- DOI: 10.1007/s40261-020-00962-y
Drug-Drug Interactions between Direct Oral Anticoagulants and Hepatitis C Direct-Acting Antiviral Agents: Looking for Evidence Through a Systematic Review
Abstract
Background: Direct oral anticoagulants (DOACs), as substrates of cytochrome P450 (CYP) 3A4 and/or P-glycoprotein, are susceptible to drug-drug interactions (DDIs). Hepatitis C direct-acting antiviral agents (DAAs), via P-glycoprotein or CYP3A4 inhibition, may increase DOAC exposure with relevant bleeding risk. We performed a systematic review on DDIs between DOACs and DAAs.
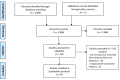
Methods: Two reviewers independently identified studies through electronic databases, until 7 July 2020, supplementing the search by reviewing conference abstracts and the ClinicalTrials.gov website.
Results: Of 1386 identified references, four articles were finally included after applying the exclusion criteria. Three phase I clinical studies in healthy volunteers assessed interactions between dabigatran and glecaprevir/pibrentasvir, odalasvir/simeprevir, or sofosbuvir/velpatasvir/voxilaprevir, showing an increase in the dabigatran area under the concentration-time curve (AUC) by 138%, 103%, and 161%, respectively.
Conclusions: DOACs and DAAs are under-investigated for DDI risk. Real-world studies are needed to assess the clinical relevance of the pharmacokinetic interactions with dabigatran and describe the actual spectrum of possible DDIs between DAAs and other DOACs.
Conflict of interest statement
Marta Bellesini, Matteo Bianchin, Chiara Corradi, and Marco Paolo Donadini declare they have no conflicts of interest. Emanuel Raschi reports personal fees from Novartis, outside the submitted work. Alessandro Squizzato received fees for lectures and/or advisory board meetings from Daiichi Sankyo, Pfizer, Bristol Myers Squibb, Bayer, and Boehringer Ingelheim.
Figures
References
-
- Ibáñez L, Sabaté M, Vidal X, et al. Incidence of direct oral anticoagulant use in patients with nonvalvular atrial fibrillation and characteristics of users in 6 European countries (2008–2015): a cross-national drug utilization study. Br J Clin Pharmacol. 2019;85:2524–2539. doi: 10.1111/bcp.14071. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical