In Vitro Effect of Different Airflow Rates on the Aerosol Properties of Nebulized Glycopyrrolate in the eFlow® Closed System and Tiotropium Delivered in the HandiHaler®
- PMID: 32809156
- PMCID: PMC7672140
- DOI: 10.1007/s41030-020-00125-6
In Vitro Effect of Different Airflow Rates on the Aerosol Properties of Nebulized Glycopyrrolate in the eFlow® Closed System and Tiotropium Delivered in the HandiHaler®
Abstract
Introduction: Personalized therapy for patients with COPD requires appropriate choice of drug and delivery device. Inhalers and nebulizers vary in their drug delivery characteristics, particularly the need for passive or active patient inhalation for appropriate drug dispersal and delivery. In this in vitro analysis, we assessed the aerosol performance and drug delivery of two long-acting muscarinic antagonists, glycopyrrolate (GLY; 25 µg solution; 1 ml) and tiotropium (TIO; 18 µg powder) through their respective delivery systems: the eFlow® Closed System (CS) vibrating membrane nebulizer and the HandiHaler® dry-powder inhaler (DPI).
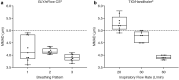
Methods: The aerosol performances of the eFlow® CS nebulizer and the HandiHaler® were determined using the Next Generation cascade Impactor. The delivered dose of GLY and TIO was determined using different breathing patterns, which varied in tidal volume and peak inspiratory flow rate, respectively, to simulate breathing conditions ranging from normal to severe obstruction.
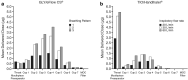
Results: Aerodynamic particle analysis showed generally similar mass median aerodynamic diameter (MMAD, range, 3.6-4.6 µm) and fine particle fraction (FPF, range, 48.2%-63.7%) with GLY delivered using the eFlow® CS nebulizer under all breathing patterns tested. TIO, delivered via the HandiHaler®, showed variations in MMAD (range, 3.8-5.8 µm) and FPF (range, 16.1%-32.4%) under different inspiratory flow rates. The majority of GLY was deposited in stages 2-5 of the impactor, which corresponds with particle sizes in the respirable range (< 5 µm), whereas a large proportion of TIO was deposited in the throat/mouthpiece pre-separator, irrespective of test conditions. The median residual dose of GLY with eFlow® CS was notably lower compared to that of TIO with HandiHaler® (2.4%-4.4% vs. 40%-67%, respectively).
Conclusions: These simulation results highlight the different deposition patterns generated by a DPI device and a vibrating membrane nebulizer, which may help inform device selection and treatment decision in COPD management.
Keywords: COPD; Dry-powder inhaler; Glycopyrrolate; Nebulizer; Tiotropium.
Figures


References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/ Accessed Jan 31 2020
-
- COPD. https://www.cdc.gov/dotw/copd/index.html. Accessed 1 Oct 2019.
LinkOut - more resources
Full Text Sources

