Impact of Interatrial Shunts on Invasive Hemodynamics and Exercise Tolerance in Patients With Heart Failure
- PMID: 32809903
- PMCID: PMC7660772
- DOI: 10.1161/JAHA.120.016760
Impact of Interatrial Shunts on Invasive Hemodynamics and Exercise Tolerance in Patients With Heart Failure
Abstract
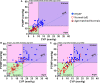
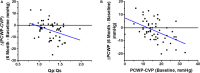
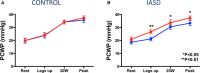
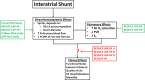
Approximately 50% of patients with heart failure have preserved ejection fraction. Although a wide variety of conditions cause or contribute to heart failure with preserved ejection fraction, elevated left ventricular filling pressures, particularly during exercise, are common to all causes. Acute elevation in left-sided filling pressures promotes lung congestion and symptoms of dyspnea, while chronic elevations often lead to pulmonary vascular remodeling, right heart failure, and increased risk of mortality. Pharmacologic therapies, including neurohormonal modulation and drugs that modify the nitric oxide/cyclic GMP-protein kinase G pathway have thus far been limited in reducing symptoms or improving outcomes in patients with heart failure with preserved ejection fraction. Hence, alternative means of reducing the detrimental rise in left-sided heart pressures are being explored. One proposed method of achieving this is to create an interatrial shunt, thus unloading the left heart at rest and during exercise. Currently available studies have shown 3- to 5-mm Hg decreases of pulmonary capillary wedge pressure during exercise despite increased workload. The mechanisms underlying the hemodynamic changes are just starting to be understood. In this review we summarize results of recent studies aimed at elucidating the potential mechanisms of improved hemodynamics during exercise tolerance following interatrial shunt implantation and the current interatrial shunt devices under investigation.
Keywords: exercise; exercise capacity; interatrial; shunt.
Conflict of interest statement
D.B. reports hemodynamic core laboratory fees from Corvia Medical, Inc. J.K. is an employee of Corvia Medical. Inc. F.G. reports consulting fees from Carmat, Abbott, Pfizer, and Boehringer‐Ingelheim, and speakers’ fees from Astra‐Zeneca, Orion Pharma. and Novartis. S.J.S. reports receiving grants from the National Institutes of Health (R01 HL140731, R01 HL120728, R01 HL107577, and R01 HL149423), the American Heart Association (#16SFRN28780016 and #15CVGPSD27260148), Actelion, AstraZeneca, Corvia, and Novartis; and has received consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Cardiora, Eisai, Ionis, Ironwood, Merck, Novartis, Pfizer, Sanofi, and United Therapeutics. The remaining authors have no disclosures to report.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

