Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal
- PMID: 32809970
- PMCID: PMC7685750
- DOI: 10.1172/JCI126584
Intestinal proinflammatory macrophages induce a phenotypic switch in interstitial cells of Cajal
Abstract
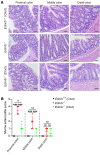
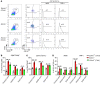
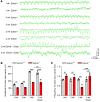
Interstitial cells of Cajal (ICCs) are pacemaker cells in the intestine, and their function can be compromised by loss of C-KIT expression. Macrophage activation has been identified in intestine affected by Hirschsprung disease-associated enterocolitis (HAEC). In this study, we examined proinflammatory macrophage activation and explored the mechanisms by which it downregulates C-KIT expression in ICCs in colon affected by HAEC. We found that macrophage activation and TNF-α production were dramatically increased in the proximal dilated colon of HAEC patients and 3-week-old Ednrb-/- mice. Moreover, ICCs lost their C-KIT+ phenotype in the dilated colon, resulting in damaged pacemaker function and intestinal dysmotility. However, macrophage depletion or TNF-α neutralization led to recovery of ICC phenotype and restored their pacemaker function. In isolated ICCs, TNF-α-mediated phosphorylation of p65 induced overexpression of microRNA-221 (miR-221), resulting in suppression of C-KIT expression and pacemaker currents. We also identified a TNF-α/NF-κB/miR-221 pathway that downregulated C-KIT expression in ICCs in the colon affected by HAEC. These findings suggest the important roles of proinflammatory macrophage activation in a phenotypic switch of ICCs, representing a promising therapeutic target for HAEC.
Keywords: Gastroenterology; Inflammatory bowel disease; Macrophages.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

