Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with chronic non-healing wound
- PMID: 32810144
- PMCID: PMC7433869
- DOI: 10.1371/journal.pone.0237746
Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with chronic non-healing wound
Abstract
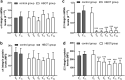
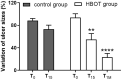
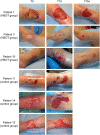
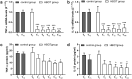
In recent years, several studies suggested that the ability of hyperbaric oxygen therapy (HBOT) to promote healing in patients with diabetic ulcers and chronic wounds is due to the reduction of inflammatory cytokines and to a significant decrease in neutrophils recruitment to the damaged area. α4 and β2 integrins are receptors mediating the neutrophil adhesion to the endothelium and the comprehension of the effects of hyperbaric oxygenation on their expression and functions in neutrophils could be of great importance for the design of novel therapeutic protocols focused on anti-inflammatory agents. In this study, the α4 and β2 integrins' expression and functions have been evaluated in human primary neutrophils obtained from patients with chronic non-healing wounds and undergoing a prolonged HBOT (150 kPa per 90 minutes). The effect of a peptidomimetic α4β1 integrin antagonist has been also analyzed under these conditions. A statistically significant decrease (68%) in β2 integrin expression on neutrophils was observed during the treatment with HBO and maintained one month after the last treatment, while α4 integrin levels remained unchanged. However, cell adhesion function of both neutrophilic integrins α4β1 and β2 was significantly reduced 70 and 67%, respectively), but α4β1 integrin was still sensitive to antagonist inhibition in the presence of fibronectin, suggesting that a combined therapy between HBOT and integrin antagonists could have greater antinflammatory efficacy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
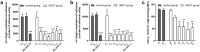

Similar articles
-
Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium.Circ Res. 2002 Mar 22;90(5):562-9. doi: 10.1161/01.res.0000013835.53611.97. Circ Res. 2002. PMID: 11909820
-
DS-70, a novel and potent α4 integrin antagonist, is an effective treatment for experimental allergic conjunctivitis in guinea pigs.Br J Pharmacol. 2018 Oct;175(20):3891-3910. doi: 10.1111/bph.14458. Epub 2018 Sep 17. Br J Pharmacol. 2018. PMID: 30051467 Free PMC article.
-
Neutrophils can adhere via alpha4beta1-integrin under flow conditions.Blood. 1997 May 15;89(10):3837-46. Blood. 1997. PMID: 9160691
-
β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function.Front Immunol. 2021 Feb 18;11:619925. doi: 10.3389/fimmu.2020.619925. eCollection 2020. Front Immunol. 2021. PMID: 33679708 Free PMC article. Review.
-
Role of β2 Integrins in Neutrophils and Sepsis.Infect Immun. 2020 May 20;88(6):e00031-20. doi: 10.1128/IAI.00031-20. Print 2020 May 20. Infect Immun. 2020. PMID: 32205406 Free PMC article. Review.
Cited by
-
Hyperbaric Oxygen Therapy as a Renewed Hope for Ischemic Craniomaxillofacial Diseases.Healthcare (Basel). 2025 Jan 13;13(2):137. doi: 10.3390/healthcare13020137. Healthcare (Basel). 2025. PMID: 39857164 Free PMC article. Review.
-
Hypoxic ischemic encephalopathy (HIE).Front Neurol. 2024 Jul 23;15:1389703. doi: 10.3389/fneur.2024.1389703. eCollection 2024. Front Neurol. 2024. PMID: 39108657 Free PMC article. Review.
-
Hyperbaric Oxygen Therapy in Systemic Inflammatory Response Syndrome.Vet Sci. 2022 Jan 18;9(2):33. doi: 10.3390/vetsci9020033. Vet Sci. 2022. PMID: 35202287 Free PMC article.
-
Diabetic Wound-Healing Science.Medicina (Kaunas). 2021 Oct 8;57(10):1072. doi: 10.3390/medicina57101072. Medicina (Kaunas). 2021. PMID: 34684109 Free PMC article. Review.
-
The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis.Biomolecules. 2021 Aug 14;11(8):1210. doi: 10.3390/biom11081210. Biomolecules. 2021. PMID: 34439876 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

