Effects of aquaporin-lipid molar ratio on the permeability of an aquaporin Z-phospholipid membrane system
- PMID: 32810188
- PMCID: PMC7437467
- DOI: 10.1371/journal.pone.0237789
Effects of aquaporin-lipid molar ratio on the permeability of an aquaporin Z-phospholipid membrane system
Abstract
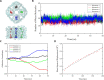
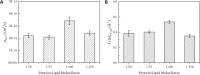
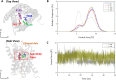
Aquaporins are water-permeable membrane-channel proteins found in biological cell membranes that selectively exclude ions and large molecules and have high water permeability, which makes them promising candidates for water desalination systems. To effectively apply the properties of aquaporins in the desalination process, many studies have been conducted on aquaporin-lipid membrane systems using phospholipids, which are the main component of cell membranes. Many parametric studies have evaluated the permeability of such systems with various aquaporin types and lipid compositions. In this study, we performed molecular dynamics simulations for four cases with different protein-lipid molar ratios (1:50, 1:75, 1:100, and 1:150) between aquaporin Z and the phospholipids, and we propose a possibility of the existence of optimal protein-lipid molar ratio to maximize water permeability. Elucidating these simulation results from a structural viewpoint suggests that there is a relationship between the permeability and changes in the hydrophobic thickness of the lipid membrane adjacent to the aquaporin as a structural parameter. The results of this study can help optimize the design of an aquaporin-lipid membrane by considering its molar ratio at an early stage of development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Evaluation of aquaporin Z water permeability in bilayers using droplet interface systems with internal-pressure-defined membrane tension.Biochim Biophys Acta Biomembr. 2025 Jun;1867(5-6):184425. doi: 10.1016/j.bbamem.2025.184425. Epub 2025 May 21. Biochim Biophys Acta Biomembr. 2025. PMID: 40409660
-
Experimental and Simulation Studies of Aquaporin 0 Water Permeability and Regulation.Chem Rev. 2019 May 8;119(9):6015-6039. doi: 10.1021/acs.chemrev.9b00106. Epub 2019 Apr 26. Chem Rev. 2019. PMID: 31026155 Review.
-
Molecular dynamics study of the archaeal aquaporin AqpM.BMC Genomics. 2011 Dec 22;12 Suppl 4(Suppl 4):S8. doi: 10.1186/1471-2164-12-S4-S8. Epub 2011 Dec 22. BMC Genomics. 2011. PMID: 22369250 Free PMC article.
-
Proton exclusion by an aquaglyceroprotein: a voltage clamp study.Biol Cell. 2005 Jul;97(7):545-50. doi: 10.1042/BC20040136. Biol Cell. 2005. PMID: 15850456
-
Water and glycerol permeation through the glycerol channel GlpF and the aquaporin family.J Synchrotron Radiat. 2004 Jan 1;11(Pt 1):86-8. doi: 10.1107/s0909049503023872. Epub 2003 Nov 28. J Synchrotron Radiat. 2004. PMID: 14646142 Review.
Cited by
-
Membrane nanodomains and transport functions in plant.Plant Physiol. 2021 Dec 4;187(4):1839-1855. doi: 10.1093/plphys/kiab312. Plant Physiol. 2021. PMID: 35235669 Free PMC article. Review.
References
-
- Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269(20):14648–14654. - PubMed
-
- Tang D, Yoo YE, Kim D. Molecular dynamics simulations on water permeation through hourglass-shaped nanopores with varying pore geometry. Chem Phys. 2015;453:13–19.
-
- Agre P, Sasaki S, Chrispeels MJ, Aquaporins: a family of water channel proteins. Am J Physiol Renal Physiol. 1993;265(3):F461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

