Performance evaluation of serological assays to determine the immunoglobulin status in SARS-CoV-2 infected patients
- PMID: 32810840
- PMCID: PMC7417972
- DOI: 10.1016/j.jcv.2020.104589
Performance evaluation of serological assays to determine the immunoglobulin status in SARS-CoV-2 infected patients
Abstract
Background: Serological assays for the determination of the immune status of patients that have tested positive for infection with SARS-CoV-2 by RT-PCR are required for, e.g., contact tracing and epidemiological studies. However, data concerning the performance parameters of commercially available high-throughput ELISA tests are still not available on a large scale.
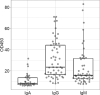
Study design: In our study, we have evaluated an in-house developed ELISA for the detection of the immunoglobulin classes A, G and M directed against the full-length spike glycoprotein from SARS-CoV-2. For this analysis, we have included 110 sera from patients presenting with COVID-19 symptoms or blood donors without symptoms collected at the Austrian Red Cross, Blood Transfusion Service for Upper Austria, Linz. In addition, we have selected four commercially available IgG-based ELISAs as well as one IgA/IgG-based ELISA for the detection of SARS-CoV-2 antigens as well as a multiplexed IgG-based micro-ELISA assay developed for rapid Point of Care testing applications.
Conclusions: All assays evaluated in the course of this study demonstrated suitable sensitivity and specificity values for the identification of patients that have experienced a past infection with SARS-CoV-2. However, testing for the presence of additional immunoglobulins (IgA and IgM) as well as using combinations of different viral antigens is highly advised to improve the predictive values of serological assays.
Keywords: COVID-19; ELISA; Evaluation; Immunoglobulin; Point of care; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
CW has been an employee of Greiner Bio-One Diagnostics GmbH from 2009 – 2016. During this time, he was member of the project team that developed the Genspeed assay platform. There is currently no professional affiliation of CW with Genspeed Biotech GmbH. The Division of Pathophysiology is serving as a reference laboratory for Genspeed Biotech GmbH within the Emergency-Call Covid-19 (FFG 880919). All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

