Cavernous carotid artery large aneurysm treated with a new flow diverter - Xcalibur aneurysm occlusion device (AOD)
- PMID: 32811244
- PMCID: PMC7645174
- DOI: 10.1177/1591019920951314
Cavernous carotid artery large aneurysm treated with a new flow diverter - Xcalibur aneurysm occlusion device (AOD)
Abstract
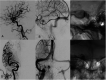
We report a case of an unruptured, symptomatic, large right cavernous internal carotid artery aneurysm successfully treated with a new balloon-expandable flow diverter - Xcalibur Aneurysm Occlusion Device (AOD). Follow up imaging performed at six months demonstrated complete exclusion of the aneurysm and regression in dimensions, resulting in resolution of mass effect and clinical improvement.
Keywords: Cavernous segment aneurysm; flow diverter; new device.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:Nihar Vijay Kathrani and Arun Kumar Gupta are Proctors of Xcalibur AOD, Merlin, MD.
Figures








Similar articles
-
Very delayed discontinuation of telescoped Pipeline embolization devices: a case report.Interv Neuroradiol. 2019 Apr;25(2):182-186. doi: 10.1177/1591019918809932. Epub 2018 Nov 4. Interv Neuroradiol. 2019. PMID: 30394838 Free PMC article.
-
Mid-term follow-up of staged bilateral internal carotid artery aneurysm treatment with Pipeline embolization.Interv Neuroradiol. 2019 Dec;25(6):664-670. doi: 10.1177/1591019919853586. Epub 2019 Jun 3. Interv Neuroradiol. 2019. PMID: 31159632 Free PMC article.
-
Flow-Diverter Stent for an Unruptured Aneurysm at the Junction of the Internal Carotid Artery and Persistent Primitive Trigeminal Artery: Case Report and Literature Review.World Neurosurg. 2019 Dec;132:329-332. doi: 10.1016/j.wneu.2019.08.199. Epub 2019 Sep 5. World Neurosurg. 2019. PMID: 31493612 Review.
-
Late recurrence of a completely occluded large intracranial aneurysm treated with a Tubridge flow diverter.BMJ Case Rep. 2016 Jun 21;2016:bcr2016012268. doi: 10.1136/bcr-2016-012268. BMJ Case Rep. 2016. PMID: 27329093 Free PMC article.
-
Treatment of Large or Giant Cavernous Aneurysm Associated with Persistent Trigeminal Artery: Case Report and Review of Literature.World Neurosurg. 2017 Dec;108:996.e11-996.e15. doi: 10.1016/j.wneu.2017.09.033. Epub 2017 Sep 14. World Neurosurg. 2017. PMID: 28919565 Review.
References
-
- Ambekar S, Madhugiri V, Sharma M, et al. Evolution of management strategies for cavernous carotid aneurysms: a review. World Neurosurg 2014; 82: 1077–1085. - PubMed
-
- Maragkos GA, Dmytriw AA, Salem MM, et al. Overview of different flow diverters and flow dynamics. Neurosurgery 2020; 86: S21–S34. - PubMed
-
- Kupersmith MJ, Stiebel-Kalish H, Huna-Baron R, et al. Cavernous carotid aneurysms rarely cause subarachnoid hemorrhage or major neurologic morbidity. J Stroke Cerebrovasc Dis 2002; 11: 9–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

