Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement
- PMID: 32811789
- PMCID: PMC7767752
- DOI: 10.1016/j.redox.2020.101674
Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement
Abstract
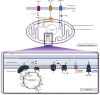
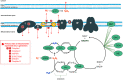
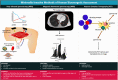
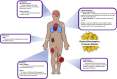
The mitochondrial electron transport chain utilizes a series of electron transfer reactions to generate cellular ATP through oxidative phosphorylation. A consequence of electron transfer is the generation of reactive oxygen species (ROS), which contributes to both homeostatic signaling as well as oxidative stress during pathology. In this graphical review we provide an overview of oxidative phosphorylation and its inter-relationship with ROS production by the electron transport chain. We also outline traditional and novel translational methodology for assessing mitochondrial energetics in health and disease.
Keywords: Electron transport chain; Mitochondria; Mitochondrial reactive oxygen species; Oxidative phosphorylation.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures

References
-
- P S. Powerhouse of the cell. Sci. Am. 1957;197(1):131–140.
-
- Jensen P.K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. II. Steroid effects. Biochim. Biophys. Acta. 1966;122(2):167–174. - PubMed
-
- Ventura-Clapier R. Bioenergetics of the failing heart. Biochim. Biophys. Acta. 2011;1813(7):1360–1372. - PubMed
-
- Stryer L. fourth ed. W.H. Freeman; New York: 1995. Biochemistry; p. 1064. xxxiv.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

