Lipoarabinomannan antigenic epitope differences in tuberculosis disease subtypes
- PMID: 32811861
- PMCID: PMC7434769
- DOI: 10.1038/s41598-020-70669-9
Lipoarabinomannan antigenic epitope differences in tuberculosis disease subtypes
Erratum in
-
Author Correction: Lipoarabinomannan antigenic epitope differences in tuberculosis disease subtypes.Sci Rep. 2021 Sep 27;11(1):19546. doi: 10.1038/s41598-021-98304-1. Sci Rep. 2021. PMID: 34580341 Free PMC article. No abstract available.
Abstract
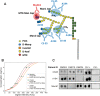
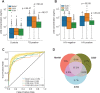
An accurate urine test for diverse populations with active tuberculosis could be transformative for preventing TB deaths. Urinary liporabinomannan (LAM) testing has been previously restricted to HIV co-infected TB patients. In this study we evaluate urinary LAM in HIV negative, pediatric and adult, pulmonary and extrapulmonary tuberculosis patients. We measured 430 microbiologically confirmed pretreatment tuberculosis patients and controls from Peru, Guinea Bissau, Venezuela, Uganda and the United States using three monoclonal antibodies, MoAb1, CS35, and A194, which recognize distinct LAM epitopes, a one-sided immunoassay, and blinded cohorts. We evaluated sources of assay variability and comorbidities (HIV and diabetes). All antibodies successfully discriminated TB positive from TB negative patients. ROAUC from the average of three antibodies' responses was 0.90; 95% CI 0.87-0.93, 90% sensitivity, 73.5% specificity (80 pg/mL). MoAb1, recognizing the 5-methylthio-D-xylofuranose(MTX)-mannose(Man) cap epitope, performed the best, was less influenced by glycosuria and identified culture positive pediatric (N = 19) and extrapulmonary (N = 24) patients with high accuracy (ROAUC 0.87, 95% CI 0.77-0.98, 0.90 sensitivity 0.80 specificity at 80 pg/mL; ROAUC = 0.96, 95% CI 0.92-0.99, 96% sensitivity, 80% specificity at 82 pg/mL, respectively). The MoAb1 antibody, recognizing the MTX-Man cap epitope, is a novel analyte for active TB detection in pediatric and extrapulmonary disease.
Conflict of interest statement
LAL, AL, and EFP are inventors on patents US9012240 and US8497137 related to the affinity particles, and AP and AC are inventors on a pending patent describing the A194 antibody. Ceres Nanosciences (Nanotrap) licensed the technology from George Mason University Research Foundation. LAL, EFP and AL own shares of Ceres Nanosciences. B.L., B.K. are Ceres Nanosciences’ employees who manufactured some reagents for this study. The other authors declare no competing interests.
Figures


References
-
- Tuberculosis (TB). https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

