Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease
- PMID: 32811965
- PMCID: PMC8209138
- DOI: 10.1038/s41401-020-0497-0
Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease
Abstract
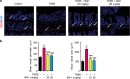
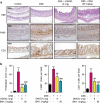
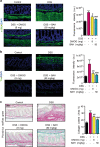
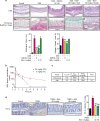
Intestinal fibrosis is a common complication of inflammatory bowel disease (IBD) and is defined as an excessive accumulation of scar tissue in the intestinal wall. Intestinal fibrosis occurs in both forms of IBD: ulcerative colitis and Crohn's disease. Small-molecule inhibitors targeting hypoxia-inducing factor (HIF) prolyl-hydroxylases are promising for the development of novel antifibrotic therapies in IBD. Herein, we evaluated the therapeutic efficacy of hydroxamate of betulinic acid (BHA), a hypoxia mimetic derivative of betulinic acid, against IBD in vitro and in vivo. We showed that BAH (5-20 μM) dose-dependently enhanced collagen gel contraction and activated the HIF pathway in NIH-3T3 fibroblasts; BAH treatment also prevented the loss of trans-epithelial electrical resistance induced by proinflammatory cytokines in Caco-2 cells. In two different murine models (TNBS- and DSS-induced IBD) that cause colon fibrosis, oral administration of BAH (20, 50 mg/kg·d, for 17 days) prevented colon inflammation and fibrosis, as detected using immunohistochemistry and qPCR assays. BAH-treated animals showed a significant reduction of fibrotic markers (Tnc, Col1a2, Col3a1, Timp-1, α-SMA) and inflammatory markers (F4/80+, CD3+, Il-1β, Ccl3) in colon tissue, as well as an improvement in epithelial barrier integrity and wound healing. BHA displayed promising oral bioavailability, no significant activity against a panel of 68 potential pharmacological targets and was devoid of genotoxicity and cardiotoxicity. Taken together, our results provide evidence that oral administration of BAH can alleviate colon inflammation and colitis-associated fibrosis, identifying the enhancement of colon barrier integrity as a possible mechanism of action, and providing a solid rationale for additional clinical studies.
Keywords: DSS; TNBS; betulinic acid hydroxamate; colon inflammation; fibrosis; hypoxia-inducible factor; inflammatory bowel disease; prolyl hydroxylases.
Conflict of interest statement
MEP, AGM, and JDUB are employees of Emerald Health Biotechnology. EM is a member of the Scientific Advisory Boards of Emerald Health Biotechnology. None of the authors have conflicts of interest. GA, AM, and EM have submitted a PCT with the publication number WO2018/069086A.
Figures





References
-
- Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16–26. - PubMed
-
- Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

