Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial
- PMID: 32812039
- PMCID: PMC7454592
- DOI: 10.1093/jac/dkaa334
Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial
Abstract
Background: Currently no effective antiviral therapy has been found to treat COVID-19. The aim of this trial was to assess if the addition of sofosbuvir and daclatasvir improved clinical outcomes in patients with moderate or severe COVID-19.
Methods: This was an open-label, multicentre, randomized controlled clinical trial in adults with moderate or severe COVID-19 admitted to four university hospitals in Iran. Patients were randomized into a treatment arm receiving sofosbuvir and daclatasvir plus standard care, or a control arm receiving standard care alone. The primary endpoint was clinical recovery within 14 days of treatment. The study is registered with IRCT.ir under registration number IRCT20200128046294N2.
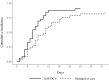
Results: Between 26 March and 26 April 2020, 66 patients were recruited and allocated to either the treatment arm (n = 33) or the control arm (n = 33). Clinical recovery within 14 days was achieved by 29/33 (88%) in the treatment arm and 22/33 (67%) in the control arm (P = 0.076). The treatment arm had a significantly shorter median duration of hospitalization [6 days (IQR 4-8)] than the control group [8 days (IQR 5-13)]; P = 0.029. Cumulative incidence of hospital discharge was significantly higher in the treatment arm versus the control (Gray's P = 0.041). Three patients died in the treatment arm and five in the control arm. No serious adverse events were reported.
Conclusions: The addition of sofosbuvir and daclatasvir to standard care significantly reduced the duration of hospital stay compared with standard care alone. Although fewer deaths were observed in the treatment arm, this was not statistically significant. Conducting larger scale trials seems prudent.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Situation Report 116 https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; doi:10.1001/jama.2020.2648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous