Spatiotemporal transformations for gaze control
- PMID: 32812395
- PMCID: PMC7435051
- DOI: 10.14814/phy2.14533
Spatiotemporal transformations for gaze control
Abstract
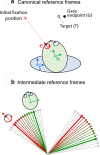
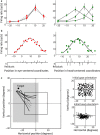
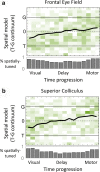
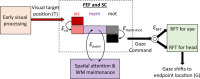
Sensorimotor transformations require spatiotemporal coordination of signals, that is, through both time and space. For example, the gaze control system employs signals that are time-locked to various sensorimotor events, but the spatial content of these signals is difficult to assess during ordinary gaze shifts. In this review, we describe the various models and methods that have been devised to test this question, and their limitations. We then describe a new method that can (a) simultaneously test between all of these models during natural, head-unrestrained conditions, and (b) track the evolving spatial continuum from target (T) to future gaze coding (G, including errors) through time. We then summarize some applications of this technique, comparing spatiotemporal coding in the primate frontal eye field (FEF) and superior colliculus (SC). The results confirm that these areas preferentially encode eye-centered, effector-independent parameters, and show-for the first time in ordinary gaze shifts-a spatial transformation between visual and motor responses from T to G coding. We introduce a new set of spatial models (T-G continuum) that revealed task-dependent timing of this transformation: progressive during a memory delay between vision and action, and almost immediate without such a delay. We synthesize the results from our studies and supplement it with previous knowledge of anatomy and physiology to propose a conceptual model where cumulative transformation noise is realized as inaccuracies in gaze behavior. We conclude that the spatiotemporal transformation for gaze is both local (observed within and across neurons in a given area) and distributed (with common signals shared across remote but interconnected structures).
© 2020 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

