Dendritic Spines: Mediators of Cognitive Resilience in Aging and Alzheimer's Disease
- PMID: 32812494
- PMCID: PMC8130863
- DOI: 10.1177/1073858420945964
Dendritic Spines: Mediators of Cognitive Resilience in Aging and Alzheimer's Disease
Abstract
Cognitive resilience is often defined as the ability to remain cognitively normal in the face of insults to the brain. These insults can include disease pathology, such as plaques and tangles associated with Alzheimer's disease, stroke, traumatic brain injury, or other lesions. Factors such as physical or mental activity and genetics may contribute to cognitive resilience, but the neurobiological underpinnings remain ill-defined. Emerging evidence suggests that dendritic spine structural plasticity is one plausible mechanism. In this review, we highlight the basic structure and function of dendritic spines and discuss how spine density and morphology change in aging and Alzheimer's disease. We note evidence that spine plasticity mediates resilience to stress, and we tackle dendritic spines in the context of cognitive resilience to Alzheimer's disease. Finally, we examine how lifestyle and genetic factors may influence dendritic spine plasticity to promote cognitive resilience before discussing evidence for actin regulatory kinases as therapeutic targets for Alzheimer's disease.
Keywords: Alzheimer’s disease; actin cytoskeleton; aging; cognitive resilience; dendritic spine.
Conflict of interest statement
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
Figures

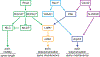
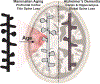
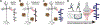
References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. 1992. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 42(3 Pt 1):631–639. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

