Extracellular Vesicles and Their Roles in Cancer Progression
- PMID: 32813249
- PMCID: PMC8008708
- DOI: 10.1007/978-1-0716-0759-6_10
Extracellular Vesicles and Their Roles in Cancer Progression
Abstract
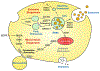
Extracellular vesicles (EVs) produced by cancer cells function as a unique form of intercellular communication that can promote cell growth and survival, help shape the tumor microenvironment, and increase invasive and metastatic activity. There are two major classes of EVs, microvesicles (MVs) and exosomes, and they differ in how they are formed. MVs are generated by the outward budding and fission of the plasma membrane. On the other hand, exosomes are derived as multivesicular bodies (MVBs) fuse with the plasma membrane and release their contents. What makes EVs especially interesting is how they mediate their effects. Both MVs and exosomes have been shown to contain a wide-variety of bioactive cargo, including cell surface, cytosolic, and nuclear proteins, as well as RNA transcripts, micro-RNAs (miRNAs), and even fragments of DNA. EVs, and their associated cargo, can be transferred to other cancer cells, as well as to normal cell types, causing the recipient cells to undergo phenotypic changes that promote different aspects of cancer progression. These findings, combined with those demonstrating that the amounts and contents of EVs produced by cancer cells can vary depending on their cell of origin, stage of development, or response to therapies, have raised the exciting possibility that EVs can be used for diagnostic purposes. Moreover, the pharmaceutical community is aggressively pursuing the use of EVs as a potential drug delivery platform. Here, in this chapter, we will highlight what is currently known about how EVs are generated, how they impact cancer progression, and the different ways they are being exploited for clinical applications.
Keywords: Exosomes; Extracellular vesicles; Intercellular communication; Microvesicles; Multivesicular bodies; Therapy deliver system; Tumor microenvironment.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

