Blood pressure and albuminuria in a female mouse model of systemic lupus erythematosus: impact of long-term high salt consumption
- PMID: 32813539
- PMCID: PMC7642904
- DOI: 10.1152/ajpregu.00070.2020
Blood pressure and albuminuria in a female mouse model of systemic lupus erythematosus: impact of long-term high salt consumption
Abstract
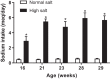
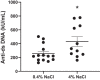
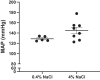
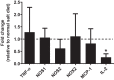
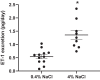
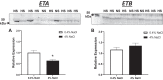
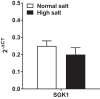
Hypertension and kidney involvement are common in patients with autoimmune disease. Sodium intake is linked to hypertension in both human and animal studies. Evidence suggests that dietary salt may be an important environmental factor that promotes autoimmune activity. Therefore, we hypothesized that a long-term high-salt diet would accelerate the progression of autoimmunity, hypertension, and albuminuria during systemic lupus erythematosus (SLE), an autoimmune disease that predominantly affects young women and has a high prevalence of hypertension and renal disease. To test this hypothesis, an established experimental model of SLE (female NZBWF1 mice) that develops hypertension and renal disease was used. SLE mice were fed a high-salt (4% NaCl) or normal (0.4% NaCl) diet for 24 wk beginning at 10 wk of age and ending at 34 wk of age, a time by which female NZBWF1 mice typically have hypertension and exhibit signs of renal disease. Plasma anti-dsDNA autoantibodies were measured as an indicator of active SLE disease, and urinary albumin was monitored longitudinally as a marker of renal disease. Arterial pressure was measured in conscious, freely moving mice at 34 wk of age. Urinary endothelin-1 (ET-1) excretion, renal endothelin A and B receptor protein expression, and renal mRNA expression of NOS1, NOS2, NOX2, MCP-1, TNF-α, serum- and glucocorticoid-regulated kinase 1, and interleukin-2 (IL-2) were assessed to determine the impact on gene products commonly altered by a high-salt diet. SLE mice fed a high-salt diet had increased circulating autoantibodies, but the high-salt diet did not significantly affect albuminuria or arterial pressure. Urinary ET-1 excretion was increased, whereas renal endothelin A receptor and IL-2 expression were decreased in response to a high-salt diet. These data suggest that a chronic high-salt diet may not accelerate cardiovascular and renal consequences commonly associated with SLE.
Keywords: autoimmunity; endothelin; hypertension; interleukin-2; systemic lupus erythematosus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Al-Herz A, Ensworth S, Shojania K, Esdaile JM. Cardiovascular risk factor screening in systemic lupus erythematosus. J Rheumatol 30: 493–496, 2003. - PubMed
-
- Boumpas DT, Austin HA III, Fessler BJ, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: emerging concepts. Part 1: Renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med 122: 940–950, 1995. doi:10.7326/0003-4819-122-12-199506150-00009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM104357/HHS | NIH | National Institute of General Medical Sciences (NIGMS)/International
- P01 HL051971/HL/NHLBI NIH HHS/United States
- HL051971/HHS | NIH | National Heart, Lung, and Blood Institute (NHBLI)/International
- P20 GM104357/GM/NIGMS NIH HHS/United States
- R01 HL134711/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

