Sex differences in the acute and subchronic lung inflammatory responses of mice to nickel nanoparticles
- PMID: 32813574
- PMCID: PMC8011295
- DOI: 10.1080/17435390.2020.1808105
Sex differences in the acute and subchronic lung inflammatory responses of mice to nickel nanoparticles
Abstract
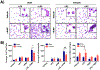
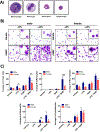
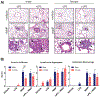
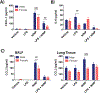
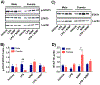
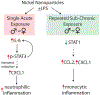
Nickel nanoparticles (NiNPs) are increasingly used in nanotechnology applications, yet information on sex differences in NiNP-induced lung disease is lacking. The goal of this study was to explore mechanisms of susceptibility between male and female mice after acute or subchronic pulmonary exposure to NiNPs. For acute exposure, male and female mice received a single dose of NiNPs with or without LPS by oropharyngeal aspiration and were necropsied 24 h later. For subchronic exposure, mice received NiNPs with or without LPS six times over 3 weeks prior to necropsy. After acute exposure to NiNPs and LPS, male mice had elevated cytokines (CXCL1 and IL-6) and more neutrophils in bronchoalveolar lavage fluid (BALF), along with greater STAT3 phosphorylation in lung tissue. After subchronic exposure to NiNPs and LPS, male mice exhibited increased monocytes in BALF. Moreover, subchronic exposure of male mice to NiNP only induced higher CXCL1 and CCL2 in BALF along with increased alveolar infiltrates and CCL2 in lung tissue. STAT1 in lung tissue was induced by subchronic exposure to NiNPs in females but not males. Males had a greater induction of IL-6 mRNA in liver after acute exposure to NiNPs and LPS, and greater CCL2 mRNA in liver after subchronic NiNP exposure. These data indicate that susceptibility of males to acute lung inflammation involves enhanced neutrophilia with increased CXCL1 and IL-6/STAT3 signaling, whereas susceptibility to subchronic lung inflammation involves enhanced monocytic infiltration with increased CXCL1 and CCL2. STAT transcription factors appear to play a role in these sex differences. This study demonstrates sex differences in the lung inflammatory response of mice to NiNPs that has implications for human disease.
Keywords: Nickel nanoparticles; inflammation; lung; sex; susceptibility.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures





Similar articles
-
Nickel nanoparticles cause exaggerated lung and airway remodeling in mice lacking the T-box transcription factor, TBX21 (T-bet).Part Fibre Toxicol. 2014 Feb 6;11:7. doi: 10.1186/1743-8977-11-7. Part Fibre Toxicol. 2014. PMID: 24499286 Free PMC article.
-
Comparative mouse lung injury by nickel nanoparticles with differential surface modification.J Nanobiotechnology. 2019 Jan 7;17(1):2. doi: 10.1186/s12951-018-0436-0. J Nanobiotechnology. 2019. PMID: 30616599 Free PMC article.
-
Synergistic induction of IL-6 production in human bronchial epithelial cells in vitro by nickel nanoparticles and lipopolysaccharide is mediated by STAT3 and C/EBPβ.Toxicol In Vitro. 2022 Sep;83:105394. doi: 10.1016/j.tiv.2022.105394. Epub 2022 May 25. Toxicol In Vitro. 2022. PMID: 35623502
-
Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications.Adv Drug Deliv Rev. 2019 Apr;144:112-132. doi: 10.1016/j.addr.2019.07.006. Epub 2019 Jul 8. Adv Drug Deliv Rev. 2019. PMID: 31295521 Free PMC article. Review.
-
Provisional Peer-Reviewed Toxicity Values for Sodium and Potassium Salts of Inorganic Phosphates (Multiple CASRNs).Cincinnati (OH): U.S. Environmental Protection Agency; 2022 Nov. Cincinnati (OH): U.S. Environmental Protection Agency; 2022 Nov. PMID: 38484098 Free Books & Documents. Review. No abstract available.
Cited by
-
Indirect mediators of systemic health outcomes following nanoparticle inhalation exposure.Pharmacol Ther. 2022 Jul;235:108120. doi: 10.1016/j.pharmthera.2022.108120. Epub 2022 Jan 24. Pharmacol Ther. 2022. PMID: 35085604 Free PMC article. Review.
-
Lung inflammation perturbation by engineered nanoparticles.Front Bioeng Biotechnol. 2023 May 25;11:1199230. doi: 10.3389/fbioe.2023.1199230. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37304133 Free PMC article. Review.
-
Sex affects the response of Wistar rats to polyvinyl pyrrolidone (PVP)-coated silver nanoparticles in an oral 28 days repeated dose toxicity study.Part Fibre Toxicol. 2021 Oct 18;18(1):38. doi: 10.1186/s12989-021-00425-y. Part Fibre Toxicol. 2021. PMID: 34663357 Free PMC article.
-
Role of the protease-activated receptor-2 (PAR2) in the exacerbation of house dust mite-induced murine allergic lung disease by multi-walled carbon nanotubes.Part Fibre Toxicol. 2023 Aug 14;20(1):32. doi: 10.1186/s12989-023-00538-6. Part Fibre Toxicol. 2023. PMID: 37580758 Free PMC article.
-
Systems level analysis of sex-dependent gene expression changes in Parkinson's disease.NPJ Parkinsons Dis. 2023 Jan 21;9(1):8. doi: 10.1038/s41531-023-00446-8. NPJ Parkinsons Dis. 2023. PMID: 36681675 Free PMC article.
References
-
- Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL and Faubel S (2012) Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. American Journal of Physiology - Renal Physiology. 303(6), pp. 864–72. doi: 10.1152/ajprenal.00025.2012. - DOI - PMC - PubMed
-
- Bonner JC, Rice AB, Lindroos PM, O’Brien PO, Dreher KL, Rosas I, Alfaro-Moreno E and Osornio-Vargas AR (1998) Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. American Journal of Respiratory Cell and Molecular Biology. 19(4), pp.672–80. doi: 10.1165/ajrcmb.19.4.3176. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
