Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia
- PMID: 32813610
- PMCID: PMC7526719
- DOI: 10.1200/JCO.20.00256
Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia
Abstract
Purpose: Nelarabine is effective in inducing remission in patients with relapsed and refractory T-cell acute lymphoblastic leukemia (T-ALL) but has not been fully evaluated in those with newly diagnosed disease.
Patients and methods: From 2007 to 2014, Children's Oncology Group trial AALL0434 (ClinicalTrials.gov identifier: NCT00408005) enrolled 1,562 evaluable patients with T-ALL age 1-31 years who received the augmented Berlin-Frankfurt-Muenster (ABFM) regimen with a 2 × 2 pseudo-factorial randomization to receive escalating-dose methotrexate (MTX) without leucovorin rescue plus pegaspargase (C-MTX) or high-dose MTX (HDMTX) with leucovorin rescue. Intermediate- and high-risk patients were also randomly assigned after induction to receive or not receive six 5-day courses of nelarabine that was incorporated into ABFM. Patients who experienced induction failure were nonrandomly assigned to HDMTX plus nelarabine. Patients with overt CNS disease (CNS3; ≥ 5 WBCs/μL with blasts) received HDMTX and were randomly assigned to receive or not receive nelarabine. All patients, except those with low-risk disease, received cranial irradiation.
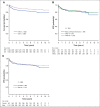
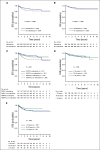
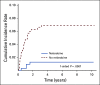
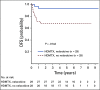
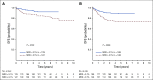
Results: The 5-year event-free and overall survival rates were 83.7% ± 1.1% and 89.5% ± 0.9%, respectively. The 5-year disease-free survival (DFS) rates for patients with T-ALL randomly assigned to nelarabine (n = 323) and no nelarabine (n = 336) were 88.2% ± 2.4% and 82.1% ± 2.7%, respectively (P = .029). Differences between DFS in a four-arm comparison were significant (P = .01), with no interactions between the MTX and nelarabine randomizations (P = .41). Patients treated with the best-performing arm, C-MTX plus nelarabine, had a 5-year DFS of 91% (n = 147). Patients who received nelarabine had significantly fewer isolated and combined CNS relapses compared with patients who did not receive nelarabine (1.3% ± 0.63% v 6.9% ± 1.4%, respectively; P = .0001). Toxicities, including neurotoxicity, were acceptable and similar between all four arms.
Conclusion: The addition of nelarabine to ABFM therapy improved DFS for children and young adults with newly diagnosed T-ALL without increased toxicity.
Figures
Comment in
-
Nelarabine in Pediatric and Young Adult T-Cell Acute Lymphoblastic Leukemia-Clearly Beneficial?J Clin Oncol. 2021 Feb 20;39(6):694. doi: 10.1200/JCO.20.02973. Epub 2021 Jan 14. J Clin Oncol. 2021. PMID: 33444071 No abstract available.
-
Reply to A. K. Agrawal et al.J Clin Oncol. 2021 Feb 20;39(6):695-696. doi: 10.1200/JCO.20.03370. Epub 2021 Jan 14. J Clin Oncol. 2021. PMID: 33444082 No abstract available.
References
-
- Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–2112. - PubMed
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

