Myeloablative haploidentical BMT with posttransplant cyclophosphamide for hematologic malignancies in children and adults
- PMID: 32813874
- PMCID: PMC7448587
- DOI: 10.1182/bloodadvances.2020001648
Myeloablative haploidentical BMT with posttransplant cyclophosphamide for hematologic malignancies in children and adults
Abstract
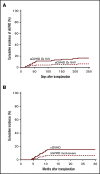
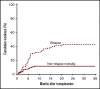
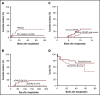
Promising results have been reported for patients with high-risk hematologic malignancies undergoing HLA-haploidentical bone marrow transplantation (haploBMT) with posttransplantation cyclophosphamide (PTCy), but there are few data on outcomes with myeloablative conditioning in this context. We report the results of a single-institution, prospective phase 2 trial of myeloablative haploBMT using busulfan-based or total body irradiation-based conditioning in 96 children or adults (median age, 42 years; range, 1-65 years) with high-risk hematologic malignancies. Recovery of neutrophils and platelets occurred at a median of 24 and 29 days. Engraftment of donor cells with chimerism >95% was achieved in 91%. The cumulative incidence of acute graft-versus-host disease (GVHD) grades II to IV and grades III to IV at day 100 was 11% and 4%, and of chronic GVHD at 6 and 12 months was 4% and 15%, with 6% moderate to severe. The cumulative incidence of nonrelapse mortality was 6% at 100 days and 11% at 1 year (19% in those aged >55 years). The cumulative incidence of relapse at 1 year was 35%; at 3 years, it was 43%. In multivariable analysis, relapse was associated with increased age (P = .02 for age 20-55 years and P = .02 for age >55 years) and with minimal residual disease before transplantation (P = .05). The overall survival at 1 and 3 years is 73% and 54%, and event-free survival at 1 and 3 years is 57% and 49%. We show that haploBMT with PTCy after myeloablative conditioning is safe and efficacious for adult and pediatric patients with hematologic malignancies. Careful consideration must be given to using myeloablative conditioning in patients age >55 years. This trial was registered at www.clinicaltrials.gov as #NCT00796562.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest-disclosure: None of the authors received any financial support for this trial. Currently, H.J.S. is on the speaker bureau for Jazz Pharmaceuticals. K.C. receives research support, is a consultant for, and is on the speaker bureau and advisory board for Jazz Pharmaceuticals. J.B.-M. is on a Drug Safety Monitoring Board for Incyte. I.G. receives research support from Merck Inc., Amgen, Amphivena, Celgene, and Genentech. L.L. receives research support from Genentech and Merck, serves on the speaker bureau for Merck, serves as a consultant and is on the advisory board for AbbVie, and is a patent holder for WindMIL Therapeutics. The remaining authors declare no competing financial interests.
Figures


References
-
- Raiola AM, Dominietto A, Ghiso A, et al. . Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117-122. - PubMed
-
- Duléry R, Bastos J, Paviglianiti A, et al. . Thiotepa, busulfan, and fludarabine conditioning regimen in T cell-replete HLA-haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(7):1407-1415. - PubMed
-
- Pagliardini T, Castagna L, Harbi S, et al. . Thiotepa, fludarabine, and busulfan conditioning regimen before T cell-replete haploidentical transplantation with post-transplant cyclophosphamide for acute myeloid leukemia: a bicentric experience of 100 patients. Biol Blood Marrow Transplant. 2019;25(9):1803-1809. - PubMed
-
- Solomon SR, Sizemore CA, Sanacore M, et al. . Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015;21(7):1299-1307. - PubMed
-
- Solomon SR, Solh M, Zhang X, Morris LE, Holland HK, Bashey A. Fludarabine and total-body irradiation conditioning before ablative haploidentical transplantation: long-term safety and efficacy. Biol Blood Marrow Transplant. 2019;25(11):2211-2216. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

