Phase II trial of S-1 treatment as palliative-intent chemotherapy for previously treated advanced thymic carcinoma
- PMID: 32813912
- PMCID: PMC7571815
- DOI: 10.1002/cam4.3385
Phase II trial of S-1 treatment as palliative-intent chemotherapy for previously treated advanced thymic carcinoma
Abstract
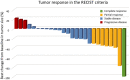
Thymic carcinoma (TC) is a rare cancer with minimal evidence of survival following palliative-intent chemotherapy. Sunitinib, everolimus, and pembrolizumab have been proposed as active agents based on previous phase II trials. In this phase II study, TC patients previously treated with platinum-based chemotherapy were enrolled. The patients received S-1 orally twice daily at a dose of 40-60 mg/m2 for 4 weeks, followed by 2 weeks off until the progression of the disease or the presence of unacceptable toxicities. The primary endpoint was the objective response rate (ORR), and secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety. The sample size of 26 patients was planned to reject the ORR of 10% under the expectation of 30% with a power of 0.80 and a type I error of 0.05 (one-sided). Twenty-six patients were recruited between 2013 and 2016; 23 patients had squamous cell carcinoma and 10 had an ECOG performance status of 0. One patient showed complete response and seven patients showed partial responses, resulting in a 30.8% response rate (90% confidence interval [CI], 18.3-46.9) and an 80.8% disease control rate (90% CI, 65.4-90.3). The median PFS was 4.3 months (95% CI, 2.3-10.3 months) and median OS was 27.4 months (95% CI, 16.6-34.3). Adverse events of grade ≥ 3 included neutropenia (12%), skin rash (8%), elevated alanine aminotransferase, and fatigue (4%). No treatment-related death was observed. S-1 confirmed clinical activity with tolerability in patients with previously treated TC. (UMIN000010736).
Keywords: S-1; chemotherapy; phase II; rare cancer; thymic carcinoma.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr Yusuke Okuma has received personal fees as honoraria from honoraria from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai, and Ono. Dr Yasushi Goto has received personal fees as honoraria from Pfizer Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Ono Pharmaceutical, Novartis Pharma, GlaxoSmithKline, and Bristol‐Myers Squibb. His institution has received research funding from Merck Serono, Pfizer Japan, Taiho Pharmaceutical, Eisai, Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Ono Pharmaceutical, Novartis Pharma, Daiichi Sankyo, GlaxoSmithKline, Yakult Honsha, Quintiles, Astellas Pharma, and Bristol‐Myers Squibb. Dr Fumiyoshi Ohyanagi has received speakers’ bureau from AstraZeneca, Novartis pharma, Daiichi Sankyo, Ono Pharmaceutical Co., LTD, Boehringer Ingelheim, Chugai Pharmaceutical.,LTD, Eli Lilly Japan KK, Taiho Pharma, MSD, and Pfizer. Kuniko Sunami, MD, PhD; Satoru Kitazono, MD, PhD; Dr Keita Kudo; Yuichi Tambo, MD; and Atsushi Horiike, MD: None declared. Dr Yoshiro Nakahara has received lecture fee from AstraZeneca, Boehringer Ingelheim, Chugai Pharmaceutical co., LTD, Eli Lilly Japan KK, Ono Pharmaceutical Co., LTD, Taiho Pharma, MSD; grant from Bristol‐Myers Squibb, Takeda, and Ono Pharmaceutical Co., LTD. Dr Shintaro Kanda has received honoraria from AstraZeneca, Ono Pharmaceutical, and BMS. Noriko Yanagitani, MD, PhD has personal fees from Chugai, Taiho Pharma, MSD, Ono, Novartis, Byer, Boehringer, outside the submitted work. Dr Hidehito Horinouchi reports grants and personal fees from BMS, grants and personal fees from Novartis, grants from Astellas, grants and personal fees from Taiho, grants and personal fees from Chugai, personal fees from Lilly, grants and personal fees from Astra Zeneca, grants and personal fees from MSD, grants from Merck serono, grants from Genomic Health, outside the submitted work. Yutaka Fujiwara, MD reports grants from Abbvie and grant and personal fees from Astra Zeneca, grant and and personal fees from BMS, grants and personal fees from Chugai, grants and personal fees from Daichi‐Sankyo, personal fees from Eisai, personal fees from Eli Lilly, grants from Incyte, grants from Merck Serono, personal fees and grants from MSD, grants and personal fees from Novartis, personal fees from Ono, outside the submitted work. Hiroshi Nokihara, MD, PhD reports grants from Pfizer and grant and personal fees from Taiho Pharmaceutical, grant from Eisai, grants and personal fees from Chugai Pharma, grants and personal fees from Eli Lily, personal fees from Novartis, personal fees from Daiichi Sankyo, grants from Glaxo SmithKleine, grants from Quintiles, grants from Astellas Pharma, personal fees and grants from AstraZeneca, grants and personal fees from Boeringer Ingelheim, grants and personal fees from Ono, personal fees from BMS, grants from Regenration, personal fee from MSD, and grants from Abbie outside the submitted work. Noboru Yamamoto, MD, PhD reports grants from Chugai, Taiho, Eisai, Lily, Quintiles, Astellas, BMS, Novartis, Daiichi‐Sankyo, Pfizer, Boefhringer Ingelheim, Kyowa‐Hakko Kirin, Bayer, Ono, Takeda, Japan Pharma, MSD, Merck, and personal fees from Ono, Chugai, AstraZeneca, Pfizer, Lilly, BMS, Eisai, Otsuka, Takeda, Boehringer Ingerheim, and Cimic., outside the submitted work. Dr Makoto Nishio has received personal fees as honoraria from Pfizer Japan, Chugai Pharmaceutical, Eli Lilly Japan, Taiho Pharmaceutical, Nichirei Biosciences, Elekta, AstraZeneca, Sanofi, Bristol‐Myers Squibb and Ono Pharmaceutical and consulting fees from Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, Taiho Pharmaceutical, Daiichi Sankyo and Pfizer Japan. His institution has received research funding from Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol‐Myers Squibb, Takeda Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Eli Lilly Japan and Pfizer Japan. Dr Yuichiro Ohe reports grants and personal fees from ONO, grants and personal fees from BMS, grants and personal fees from AstraZeneca, grants and personal fees from Chugai, grants and personal fees from Lilly, grants and personal fees from Taiho, grants and personal fees from Takeda, grants and personal fees from Novartis, personal fees from Kissei grants and personal fees from Pfizer, grants and personal fees from MSD, grants from Jassen, grants from LOXO Oncology, grants and personal fees from Kyorin, grants from Dainippon‐Sumitomo, personal fees from Celtrion, personal fees from Kyowa Hakko Kirin, personal fees from Amgen, personal fees from Nippon Kayaku personal fees from Boehringer Ingelheim, personal fees from Bayer and grants from Ignyta, outside the submitted work. Dr Yukio Hosomi reports personal fees from AstraZeneca, personal fees from Eli Lilly Japan, personal fees from Taiho Pharmaceutical, personal fees from Chugai Pharmaceutical, personal fees from Ono Pharmaceutical, personal fees from Bristol‐Myers Squibb, personal fees from Kyowa Kirin, personal fees from CSL Behring, outside the submitted work. No other disclosures are reported.
Figures


References
-
- Casali PG. Rare cancers: work in progress in Europe. Ann Oncol. 2014;25:914. - PubMed
-
- Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493‐2511. - PubMed
-
- Rare Cancers Europe. https://www.rarecancerseurope.org/. Accessed August 10, 2020.
-
- Girard N, Ruffini E, Marx A, Faivre‐Finn C, Peters S, Committee EG. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v40‐55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

