Multiscale Simulation Reveals Passive Proton Transport Through SERCA on the Microsecond Timescale
- PMID: 32814059
- PMCID: PMC7474205
- DOI: 10.1016/j.bpj.2020.07.027
Multiscale Simulation Reveals Passive Proton Transport Through SERCA on the Microsecond Timescale
Abstract
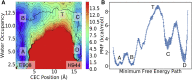
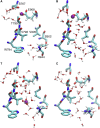
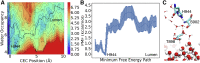
The sarcoplasmic reticulum Ca2+-ATPase (SERCA) transports two Ca2+ ions from the cytoplasm to the reticulum lumen at the expense of ATP hydrolysis. In addition to transporting Ca2+, SERCA facilitates bidirectional proton transport across the sarcoplasmic reticulum to maintain the charge balance of the transport sites and to balance the charge deficit generated by the exchange of Ca2+. Previous studies have shown the existence of a transient water-filled pore in SERCA that connects the Ca2+ binding sites with the lumen, but the capacity of this pathway to sustain passive proton transport has remained unknown. In this study, we used the multiscale reactive molecular dynamics method and free energy sampling to quantify the free energy profile and timescale of the proton transport across this pathway while also explicitly accounting for the dynamically coupled hydration changes of the pore. We find that proton transport from the central binding site to the lumen has a microsecond timescale, revealing a novel passive cytoplasm-to-lumen proton flow beside the well-known inverse proton countertransport occurring in active Ca2+ transport. We propose that this proton transport mechanism is operational and serves as a functional conduit for passive proton transport across the sarcoplasmic reticulum.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
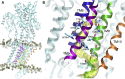
Comment in
-
A Computational Swiss Army Knife Approach to Unraveling the Secrets of Proton Movement through SERCA.Biophys J. 2020 Sep 1;119(5):890-891. doi: 10.1016/j.bpj.2020.07.028. Epub 2020 Aug 6. Biophys J. 2020. PMID: 32795397 Free PMC article. No abstract available.
References
-
- Levy D., Seigneuret M., Rigaud J.L. Evidence for proton countertransport by the sarcoplasmic reticulum Ca2+-ATPase during calcium transport in reconstituted proteoliposomes with low ionic permeability. J. Biol. Chem. 1990;265:19524–19534. - PubMed
-
- Forge V., Mintz E., Guillain F. Ca2+ binding to sarcoplasmic reticulum ATPase revisited. I. Mechanism of affinity and cooperativity modulation by H+ and Mg2+ J. Biol. Chem. 1993;268:10953–10960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

