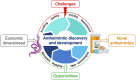
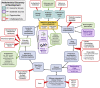
Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics
- PMID: 32814269
- PMCID: PMC7452592
- DOI: 10.1016/j.ijpddr.2020.07.001
Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics
Abstract
Control of helminth parasites is a key challenge for human and veterinary medicine. In the absence of effective vaccines and adequate sanitation, prophylaxis and treatment commonly rely upon anthelmintics. There are concerns about the development of drug resistance, side-effects, lack of efficacy and cost-effectiveness that drive the need for new classes of anthelmintics. Despite this need, only three new drug classes have reached the animal market since 2000 and no new classes of anthelmintic have been approved for human use. So where are all the anthelmintics? What are the barriers to anthelmintic discovery, and what emerging opportunities can be used to address this? This was a discussion group focus at the 2019 8th Consortium for Anthelmintic Resistance and Susceptibility (CARS) in Wisconsin, USA. Here we report the findings of the group in the broader context of the human and veterinary anthelmintic discovery pipeline, highlighting challenges unique to antiparasitic drug discovery. We comment on why the development of novel anthelmintics has been so rare. Further, we discuss potential opportunities for drug development moving into the 21st Century.
Keywords: Anthelmintics; Challenges; Drug discovery; Helminths; Nematodes; Opportunities.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors are employees of different universities, federal institutions and independent commercial companies as listed below. The opinions expressed by the authors were written in good faith for the scientific benefit.
Figures
Similar articles
-
Foreword: towards markers for anthelmintic resistance in helminths of importance in animal and human health.Parasitology. 2007;134(Pt 8):1073-6. doi: 10.1017/S0031182007000078. Parasitology. 2007. PMID: 17608966
-
Whole-organism phenotypic screening methods used in early-phase anthelmintic drug discovery.Biotechnol Adv. 2022 Jul-Aug;57:107937. doi: 10.1016/j.biotechadv.2022.107937. Epub 2022 Mar 7. Biotechnol Adv. 2022. PMID: 35271946 Review.
-
Managing anthelmintic resistance--parasite fitness, drug use strategy and the potential for reversion towards susceptibility.Vet Parasitol. 2013 Nov 15;198(1-2):145-53. doi: 10.1016/j.vetpar.2013.08.022. Epub 2013 Aug 29. Vet Parasitol. 2013. PMID: 24074608
-
Will technology provide solutions for drug resistance in veterinary helminths?Vet Parasitol. 2005 Sep 30;132(3-4):223-39. doi: 10.1016/j.vetpar.2005.07.014. Vet Parasitol. 2005. PMID: 16118040 Review.
-
Present-day anthelmintics and perspectives on future new targets.Parasitol Res. 2014 Jul;113(7):2425-33. doi: 10.1007/s00436-014-3969-7. Epub 2014 Jun 4. Parasitol Res. 2014. PMID: 24894082 Review.
Cited by
-
Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets.Int J Mol Sci. 2025 Mar 28;26(7):3134. doi: 10.3390/ijms26073134. Int J Mol Sci. 2025. PMID: 40243899 Free PMC article.
-
A validated high-throughput method for assaying rat lungworm (Angiostrongylus cantonensis) motility when challenged with potentially anthelmintic natural products from Hawaiian fungi.Parasitology. 2022 Mar 3;149(6):1-28. doi: 10.1017/S0031182022000191. Online ahead of print. Parasitology. 2022. PMID: 35236524 Free PMC article.
-
Global impact of parasitic infections and the importance of parasite control.Front Parasitol. 2025 Mar 10;4:1546195. doi: 10.3389/fpara.2025.1546195. eCollection 2025. Front Parasitol. 2025. PMID: 40129690 Free PMC article. Review.
-
A new class of natural anthelmintics targeting lipid metabolism.Nat Commun. 2025 Jan 2;16(1):305. doi: 10.1038/s41467-024-54965-w. Nat Commun. 2025. PMID: 39746976 Free PMC article.
-
New Insights into Anthelmintic Mechanisms of Action of a Synthetic Peptide: An Ultrastructural and Nanomechanical Approach.Polymers (Basel). 2021 Jul 20;13(14):2370. doi: 10.3390/polym13142370. Polymers (Basel). 2021. PMID: 34301127 Free PMC article.
References
-
- Alonso D., Munoz J., Gascon J., Valls M.E., Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 2006;74:342–344. - PubMed
-
- Alvarez-Bujidos L., Ortiz A.I., Molina-Martinez I.T., Cubria C., Ordonez D. Pharmacokinetics of intravenous luxabendazole in rabbits: influence of the enterohepatic circulation. Biopharm Drug Dispos. 1998;19:341–347. - PubMed
-
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M., Schuster D., Breuss J.M., Bochkov V., Mihovilovic M.D., Kopp B., Bauer R., Dirsch V.M., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. - PMC - PubMed
-
- Aulner N., Danckaert A., Ihm J., Shum D., Shorte S.L. Next-generation phenotypic screening in early drug discovery for infectious diseases. Trends Parasitol. 2019;35:559–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources