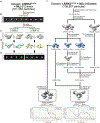
Structure of LRRK2 in Parkinson's disease and model for microtubule interaction
- PMID: 32814344
- PMCID: PMC7726071
- DOI: 10.1038/s41586-020-2673-2
Structure of LRRK2 in Parkinson's disease and model for microtubule interaction
Abstract
Leucine-rich repeat kinase 2 (LRRK2) is the most commonly mutated gene in familial Parkinson's disease1 and is also linked to its idiopathic form2. LRRK2 has been proposed to function in membrane trafficking3 and colocalizes with microtubules4. Despite the fundamental importance of LRRK2 for understanding and treating Parkinson's disease, structural information on the enzyme is limited. Here we report the structure of the catalytic half of LRRK2, and an atomic model of microtubule-associated LRRK2 built using a reported cryo-electron tomography in situ structure5. We propose that the conformation of the LRRK2 kinase domain regulates its interactions with microtubules, with a closed conformation favouring oligomerization on microtubules. We show that the catalytic half of LRRK2 is sufficient for filament formation and blocks the motility of the microtubule-based motors kinesin 1 and cytoplasmic dynein 1 in vitro. Kinase inhibitors that stabilize an open conformation relieve this interference and reduce the formation of LRRK2 filaments in cells, whereas inhibitors that stabilize a closed conformation do not. Our findings suggest that LRRK2 can act as a roadblock for microtubule-based motors and have implications for the design of therapeutic LRRK2 kinase inhibitors.
Figures












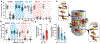
Comment in
-
"Opening" New Insights Into LRRK2 Conformation and the Microtubule.Mov Disord. 2020 Dec;35(12):2162-2163. doi: 10.1002/mds.28351. Epub 2020 Oct 21. Mov Disord. 2020. PMID: 33085790 No abstract available.
References
-
- Monfrini E & Di Fonzo A Leucine-Rich Repeat Kinase (LRRK2) Genetics and Parkinson’s Disease. Adv Neurobiol 14, 3–30 (2017). - PubMed
-
- Abeliovich A & Gitler AD Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539, 207–216 (2016). - PubMed
-
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O’Neill E, Meitinger T, Kolch W, Prokisch H & Ueffing M The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 15, 223–232 (2006). - PubMed
METHODS AND EXTENDED DATA REFERENCES
-
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC & Ferrin TE UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004). - PubMed
-
- Charrier J-D, Miller A, Kay DP, Brenchley G, Twin HC, Collier PN, Ramaya S, Keily SB, Durrant SJ, Knegtel RMA, Tanner AJ, Brown K, Curnock AP & Jimenez J-M Discovery and structure-activity relationship of 3-aminopyrid-2-ones as potent and selective interleukin-2 inducible T-cell kinase (Itk) inhibitors. J Med Chem 54, 2341–2350 (2011). - PubMed
-
- Woehlke G, Ruby AK, Hart CL, Ly B, Hom-Booher N & Vale RD Microtubule interaction site of the kinesin motor. Cell 90, 207–216 (1997). - PubMed
-
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS & Carragher B Automated molecular microscopy: the new Leginon system. J Struct Biol 151, 41–60 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

