Seasonal influenza vaccination in Kenya: an economic evaluation using dynamic transmission modelling
- PMID: 32814581
- PMCID: PMC7438179
- DOI: 10.1186/s12916-020-01687-7
Seasonal influenza vaccination in Kenya: an economic evaluation using dynamic transmission modelling
Abstract
Background: There is substantial burden of seasonal influenza in Kenya, which led the government to consider introducing a national influenza vaccination programme. Given the cost implications of a nationwide programme, local economic evaluation data are needed to inform policy on the design and benefits of influenza vaccination. We set out to estimate the cost-effectiveness of seasonal influenza vaccination in Kenya.
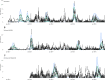
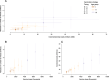
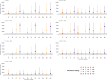
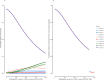
Methods: We fitted an age-stratified dynamic transmission model to active surveillance data from patients with influenza from 2010 to 2018. Using a societal perspective, we developed a decision tree cost-effectiveness model and estimated the incremental cost-effectiveness ratio (ICER) per disability-adjusted life year (DALY) averted for three vaccine target groups: children 6-23 months (strategy I), 2-5 years (strategy II) and 6-14 years (strategy III) with either the Southern Hemisphere influenza vaccine (Strategy A) or Northern Hemisphere vaccine (Strategy B) or both (Strategy C: twice yearly vaccination campaigns, or Strategy D: year-round vaccination campaigns). We assessed cost-effectiveness by calculating incremental net monetary benefits (INMB) using a willingness-to-pay (WTP) threshold of 1-51% of the annual gross domestic product per capita ($17-$872).
Results: The mean number of infections across all ages was 2-15 million per year. When vaccination was well timed to influenza activity, the annual mean ICER per DALY averted for vaccinating children 6-23 months ranged between $749 and $1385 for strategy IA, $442 and $1877 for strategy IB, $678 and $4106 for strategy IC and $1147 and $7933 for strategy ID. For children 2-5 years, it ranged between $945 and $1573 for strategy IIA, $563 and $1869 for strategy IIB, $662 and $4085 for strategy IIC, and $1169 and $7897 for strategy IID. For children 6-14 years, it ranged between $923 and $3116 for strategy IIIA, $1005 and $2223 for strategy IIIB, $883 and $4727 for strategy IIIC and $1467 and $6813 for strategy IIID. Overall, no vaccination strategy was cost-effective at the minimum ($17) and median ($445) WTP thresholds. Vaccinating children 6-23 months once a year had the highest mean INMB value at $872 (WTP threshold upper limit); however, this strategy had very low probability of the highest net benefit.
Conclusion: Vaccinating children 6-23 months once a year was the most favourable vaccination option; however, the strategy is unlikely to be cost-effective given the current WTP thresholds.
Keywords: Cost-effectiveness; Dynamic transmission model; Economic evaluation; Influenza vaccine; Low- and middle-income countries; Vaccine target group; Vaccine timing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Office of Director of Medical Services Ministry of Health . Communication to stakeholders: Progress update on plans to introduce influenza vaccine into routine immunization in Kenya. Nairobi: Ministry of Health, Kenya; 2016.
-
- Dawa J, Chaves SS, Ba Nguz A, Kalani R, Anyango E, Mutie D, et al. Developing a seasonal influenza vaccine recommendation in Kenya: process and challenges faced by the National Immunization Technical Advisory Group (NITAG) Vaccine. 2019;37(3):464–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

