CD28 costimulation drives tumor-infiltrating T cell glycolysis to promote inflammation
- PMID: 32814710
- PMCID: PMC7455120
- DOI: 10.1172/jci.insight.138729
CD28 costimulation drives tumor-infiltrating T cell glycolysis to promote inflammation
Abstract
Metabolic reprogramming dictates the fate and function of stimulated T cells, yet these pathways can be suppressed in T cells in tumor microenvironments. We previously showed that glycolytic and mitochondrial adaptations directly contribute to reducing the effector function of renal cell carcinoma (RCC) CD8+ tumor-infiltrating lymphocytes (TILs). Here we define the role of these metabolic pathways in the activation and effector functions of CD8+ RCC TILs. CD28 costimulation plays a key role in augmenting T cell activation and metabolism, and is antagonized by the inhibitory and checkpoint immunotherapy receptors CTLA4 and PD-1. While RCC CD8+ TILs were activated at a low level when stimulated through the T cell receptor alone, addition of CD28 costimulation greatly enhanced activation, function, and proliferation. CD28 costimulation reprogrammed RCC CD8+ TIL metabolism with increased glycolysis and mitochondrial oxidative metabolism, possibly through upregulation of GLUT3. Mitochondria also fused to a greater degree, with higher membrane potential and overall mass. These phenotypes were dependent on glucose metabolism, as the glycolytic inhibitor 2-deoxyglucose both prevented changes to mitochondria and suppressed RCC CD8+ TIL activation and function. These data show that CD28 costimulation can restore RCC CD8+ TIL metabolism and function through rescue of T cell glycolysis that supports mitochondrial mass and activity.
Keywords: Glucose metabolism; Immunology; Immunotherapy; Oncology; T cells.
Conflict of interest statement
Figures

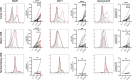
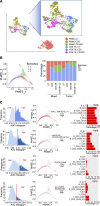

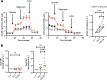
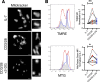
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

