Lack of Kcnn4 improves mucociliary clearance in muco-obstructive lung disease
- PMID: 32814712
- PMCID: PMC7455130
- DOI: 10.1172/jci.insight.140076
Lack of Kcnn4 improves mucociliary clearance in muco-obstructive lung disease
Abstract
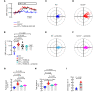
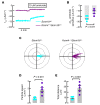
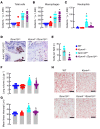
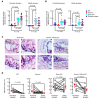
Airway mucociliary clearance (MCC) is the main mechanism of lung defense keeping airways free of infection and mucus obstruction. Airway surface liquid volume, ciliary beating, and mucus are central for proper MCC and critically regulated by sodium absorption and anion secretion. Impaired MCC is a key feature of muco-obstructive diseases. The calcium-activated potassium channel KCa.3.1, encoded by Kcnn4, participates in ion secretion, and studies showed that its activation increases Na+ absorption in airway epithelia, suggesting that KCa3.1-induced hyperpolarization was sufficient to drive Na+ absorption. However, its role in airway epithelium is not fully understood. We aimed to elucidate the role of KCa3.1 in MCC using a genetically engineered mouse. KCa3.1 inhibition reduced Na+ absorption in mouse and human airway epithelium. Furthermore, the genetic deletion of Kcnn4 enhanced cilia beating frequency and MCC ex vivo and in vivo. Kcnn4 silencing in the Scnn1b-transgenic mouse (Scnn1btg/+), a model of muco-obstructive lung disease triggered by increased epithelial Na+ absorption, improved MCC, reduced Na+ absorption, and did not change the amount of mucus but did reduce mucus adhesion, neutrophil infiltration, and emphysema. Our data support that KCa3.1 inhibition attenuated muco-obstructive disease in the Scnn1btg/+ mice. K+ channel modulation may be a therapeutic strategy to treat muco-obstructive lung diseases.
Keywords: Epithelial transport of ions and water; Inflammation; Potassium channels; Pulmonology.
Conflict of interest statement
Figures

Similar articles
-
Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models.J Aerosol Med Pulm Drug Deliv. 2008 Mar;21(1):13-24. doi: 10.1089/jamp.2007.0659. J Aerosol Med Pulm Drug Deliv. 2008. PMID: 18518828 Review.
-
Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction.Physiol Genomics. 2012 Apr 15;44(8):470-84. doi: 10.1152/physiolgenomics.00185.2011. Epub 2012 Mar 6. Physiol Genomics. 2012. PMID: 22395316 Free PMC article.
-
SNSP113 (PAAG) improves mucociliary transport and lung pathology in the Scnn1b-Tg murine model of CF lung disease.J Cyst Fibros. 2023 Nov;22(6):1104-1112. doi: 10.1016/j.jcf.2023.08.011. Epub 2023 Sep 14. J Cyst Fibros. 2023. PMID: 37714777 Free PMC article.
-
Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases.Cell Tissue Res. 2017 Mar;367(3):537-550. doi: 10.1007/s00441-016-2562-z. Epub 2017 Jan 20. Cell Tissue Res. 2017. PMID: 28108847 Review.
-
Impaired mucus clearance exacerbates allergen-induced type 2 airway inflammation in juvenile mice.J Allergy Clin Immunol. 2017 Jul;140(1):190-203.e5. doi: 10.1016/j.jaci.2016.09.045. Epub 2016 Nov 16. J Allergy Clin Immunol. 2017. PMID: 27865862
Cited by
-
Epigenetic regulation of macrophage activation in chronic obstructive pulmonary disease.Front Immunol. 2024 Aug 14;15:1445372. doi: 10.3389/fimmu.2024.1445372. eCollection 2024. Front Immunol. 2024. PMID: 39206196 Free PMC article. Review.
-
Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis.Cell Mol Immunol. 2022 Aug;19(8):925-943. doi: 10.1038/s41423-022-00891-0. Epub 2022 Jul 7. Cell Mol Immunol. 2022. PMID: 35799057 Free PMC article.
-
Cyclosporine A Accelerates Neurorecovery Transcriptional Trajectory in a Swine Model of Diffuse Traumatic Brain Injury.Int J Mol Sci. 2025 Apr 9;26(8):3531. doi: 10.3390/ijms26083531. Int J Mol Sci. 2025. PMID: 40331981 Free PMC article.
-
Inhibition of the sodium-dependent HCO3- transporter SLC4A4, produces a cystic fibrosis-like airway disease phenotype.Elife. 2022 May 30;11:e75871. doi: 10.7554/eLife.75871. Elife. 2022. PMID: 35635440 Free PMC article.
-
TRPV4 Channel Modulators as Potential Drug Candidates for Cystic Fibrosis.Int J Mol Sci. 2024 Sep 30;25(19):10551. doi: 10.3390/ijms251910551. Int J Mol Sci. 2024. PMID: 39408877 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

