Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections
- PMID: 32814862
- PMCID: PMC8559572
- DOI: 10.1038/s41579-020-0420-1
Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections
Abstract
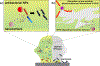
Antibiotic-resistant bacterial infections arising from acquired resistance and/or through biofilm formation necessitate the development of innovative 'outside of the box' therapeutics. Nanomaterial-based therapies are promising tools to combat bacterial infections that are difficult to treat, featuring the capacity to evade existing mechanisms associated with acquired drug resistance. In addition, the unique size and physical properties of nanomaterials give them the capability to target biofilms, overcoming recalcitrant infections. In this Review, we highlight the general mechanisms by which nanomaterials can be used to target bacterial infections associated with acquired antibiotic resistance and biofilms. We emphasize design elements and properties of nanomaterials that can be engineered to enhance potency. Lastly, we present recent progress and remaining challenges for widespread clinical implementation of nanomaterials as antimicrobial therapeutics.
Conflict of interest statement
Competing Interests Statement
Dr. Patel reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics Limited and Shionogi. Dr. Patel is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics and Qvella; monies are paid to Mayo Clinic. In addition, Dr. Patel has a patent on
Figures


Comment in
-
Nanomaterials arising amid antibiotic resistance.Nat Rev Microbiol. 2021 Jan;19(1):5-6. doi: 10.1038/s41579-020-00469-5. Nat Rev Microbiol. 2021. PMID: 33024312 Free PMC article.
References
-
- CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; (2019).
-
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report. WHO; (2017). ISBN 978-92-4-151344-9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

