A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases
- PMID: 32814863
- PMCID: PMC7784893
- DOI: 10.1038/s41396-020-00744-6
A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases
Abstract
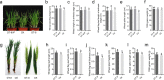
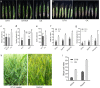
Fungal pathogens are seriously threatening food security and natural ecosystems; efficient and environmentally friendly control methods are essential to help safeguard such resources for increasing human populations on a global scale. Here, we find that Sclerotinia sclerotiorum, a widespread pathogen of dicotyledons, can grow endophytically in wheat, rice, barley, maize, and oat, providing protection against Fusarium head blight, stripe rust, and rice blast. Protection is also provided by disabled S. sclerotiorum strains harboring a hypovirulence virus. The disabled strain DT-8 promoted wheat yields by 4-18% in the field and consistently reduced Fusarium disease by 40-60% across multiple field trials. We term the host-dependent trophism of S. sclerotiorum, destructively pathogenic or mutualistically endophytic, as schizotrophism. As a biotroph, S. sclerotiorum modified the expression of wheat genes involved in disease resistance and photosynthesis and increased the level of IAA. Our study shows that a broad-spectrum pathogen of one group of plants may be employed as a biocontrol agent in a different group of plants where they can be utilized as beneficial microorganisms while avoiding the risk of in-field release of pathogens. Our study also raises provocative questions about the potential role of schizotrophic endophytes in natural ecosystems.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Schizotrophic Sclerotinia sclerotiorum-Mediated Root and Rhizosphere Microbiome Alterations Activate Growth and Disease Resistance in Wheat.Microbiol Spectr. 2023 Jun 15;11(3):e0098123. doi: 10.1128/spectrum.00981-23. Epub 2023 May 22. Microbiol Spectr. 2023. PMID: 37212718 Free PMC article.
-
The schizotrophic lifestyle of Sclerotinia sclerotiorum.Mol Plant Pathol. 2024 Feb;25(2):e13423. doi: 10.1111/mpp.13423. Mol Plant Pathol. 2024. PMID: 38407560 Free PMC article. Review.
-
Exploring the Mycovirus Sclerotinia sclerotiorum Hypovirulence-Associated DNA Virus 1 as a Biocontrol Agent of White Mold Caused by Sclerotinia sclerotiorum.Plant Dis. 2024 Mar;108(3):624-634. doi: 10.1094/PDIS-07-23-1458-RE. Epub 2024 Mar 4. Plant Dis. 2024. PMID: 37743591
-
Transcriptional plasticity of schizotrophic Sclerotinia sclerotiorum responds to symptomatic rapeseed and endophytic wheat hosts.Microbiol Spectr. 2023 Dec 12;11(6):e0261223. doi: 10.1128/spectrum.02612-23. Epub 2023 Oct 31. Microbiol Spectr. 2023. PMID: 37905914 Free PMC article.
-
Viruses of plant-pathogenic fungi: a promising biocontrol strategy for Sclerotinia sclerotiorum.Arch Microbiol. 2023 Dec 24;206(1):38. doi: 10.1007/s00203-023-03774-8. Arch Microbiol. 2023. PMID: 38142438 Review.
Cited by
-
Rational management of the plant microbiome for the Second Green Revolution.Plant Commun. 2024 Apr 8;5(4):100812. doi: 10.1016/j.xplc.2024.100812. Epub 2024 Jan 11. Plant Commun. 2024. PMID: 38213028 Free PMC article. Review.
-
Distinct effects of phyllosphere and rhizosphere microbes on invader Ageratina adenophora during its early life stages.Elife. 2024 Jun 19;13:RP95502. doi: 10.7554/eLife.95502. Elife. 2024. PMID: 38896455 Free PMC article.
-
Manipulating the plant mycobiome to enhance resilience: Ecological and evolutionary opportunities and challenges.PLoS Pathog. 2023 Dec 14;19(12):e1011816. doi: 10.1371/journal.ppat.1011816. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38096141 Free PMC article. No abstract available.
-
A Novel Heptasegmented Positive-Sense Single-Stranded RNA Virus from the Phytopathogenic Fungus Colletotrichum fructicola.J Virol. 2022 May 11;96(9):e0031822. doi: 10.1128/jvi.00318-22. Epub 2022 Apr 18. J Virol. 2022. PMID: 35435725 Free PMC article.
-
Nine viruses from eight lineages exhibiting new evolutionary modes that co-infect a hypovirulent phytopathogenic fungus.PLoS Pathog. 2021 Aug 24;17(8):e1009823. doi: 10.1371/journal.ppat.1009823. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34428260 Free PMC article.
References
-
- Agrios GN. Plant pathology. 5th ed. Elsevier Academic Press; Theobaldʼs Road, London WC1X 8RR, UK, 2005.
-
- Wu F. Perspective: time to face the fungal threat. Nature. 2014;516:S7. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

