An intramembrane chaperone complex facilitates membrane protein biogenesis
- PMID: 32814900
- PMCID: PMC7610463
- DOI: 10.1038/s41586-020-2624-y
An intramembrane chaperone complex facilitates membrane protein biogenesis
Abstract
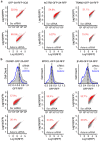
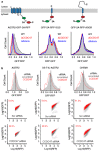
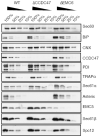
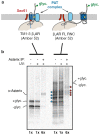
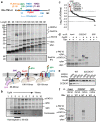
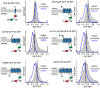
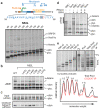
Integral membrane proteins are encoded by approximately 25% of all protein-coding genes1. In eukaryotes, the majority of membrane proteins are inserted, modified and folded at the endoplasmic reticulum (ER)2. Research over the past several decades has determined how membrane proteins are targeted to the ER and how individual transmembrane domains (TMDs) are inserted into the lipid bilayer3. By contrast, very little is known about how multi-spanning membrane proteins with several TMDs are assembled within the membrane. During the assembly of TMDs, interactions between polar or charged amino acids typically stabilize the final folded configuration4-8. TMDs with hydrophilic amino acids are likely to be chaperoned during the co-translational biogenesis of membrane proteins; however, ER-resident intramembrane chaperones are poorly defined. Here we identify the PAT complex, an abundant obligate heterodimer of the widely conserved ER-resident membrane proteins CCDC47 and Asterix. The PAT complex engages nascent TMDs that contain unshielded hydrophilic side chains within the lipid bilayer, and it disengages concomitant with substrate folding. Cells that lack either subunit of the PAT complex show reduced biogenesis of numerous multi-spanning membrane proteins. Thus, the PAT complex is an intramembrane chaperone that protects TMDs during assembly to minimize misfolding of multi-spanning membrane proteins and maintain cellular protein homeostasis.
Conflict of interest statement
Figures



Comment in
-
PAT in the ER for Transmembrane Protein Folding.Trends Biochem Sci. 2020 Dec;45(12):1007-1008. doi: 10.1016/j.tibs.2020.10.001. Epub 2020 Oct 17. Trends Biochem Sci. 2020. PMID: 33082068
-
Membrane Protein Biogenesis: PAT Complex Pats Membrane Proteins into Shape.Curr Biol. 2020 Nov 16;30(22):R1387-R1389. doi: 10.1016/j.cub.2020.09.072. Curr Biol. 2020. PMID: 33202243
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

