Analytical and Clinical Evaluation of the Automated Elecsys Anti-SARS-CoV-2 Antibody Assay on the Roche cobas e602 Analyzer
- PMID: 32814955
- PMCID: PMC7454296
- DOI: 10.1093/ajcp/aqaa155
Analytical and Clinical Evaluation of the Automated Elecsys Anti-SARS-CoV-2 Antibody Assay on the Roche cobas e602 Analyzer
Abstract
Objectives: To evaluate the analytical and clinical performance of the automated Elecsys anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody (Elecsys Ab) assay on the Roche cobas e602 analyzer. With the ongoing global coronavirus disease 2019 (COVID-19) pandemic, widespread and routine serologic testing of SARS-CoV-2 remains a pressing need. To better understand its epidemiologic spread and to support policies aimed at curtailing further infections, reliable serologic testing is crucial for providing insight into the dynamics of the spread of COVID-19 on a population level.
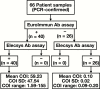
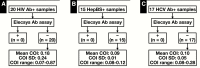
Methods: The presence of anti-SARS-CoV-2 antibodies in polymerase chain reaction-positive, confirmed COVID-19 patient samples was determined using the Elecsys Ab assay on the Roche cobas e602 analyzer. The precision and cross-reactivity of the Elecsys Ab assay were characterized and its performance was compared against the EuroImmun IgA/IgG antibody (EuroImmun Ab) assay. Calculated sensitivity, specificity, and positive and negative predictive values were assessed.
Results: The Elecsys Ab assay demonstrated good precision, had no cross-reactivity with other viral samples, and showed 100% concordance with the EuroImmun Ab assay. Excellent clinical performance with respect to sensitivity, specificity, and positive and negative predictive values was observed.
Conclusions: The Elecsys Ab assay is a precise and highly reliable automated platform for clinical detection of seropositivity in SARS-CoV-2 infection.
Keywords: COVID-19; Coronavirus; Diagnostics; IgA; IgG; IgM; Immunoassay; SAR-CoV-2 antibodies; Serology; Surveillance.
© American Society for Clinical Pathology, 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Kamel Boulos MN, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int J Health Geogr. 2020;19:8. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

