The cryo-EM structure of the human uromodulin filament core reveals a unique assembly mechanism
- PMID: 32815518
- PMCID: PMC7486124
- DOI: 10.7554/eLife.60265
The cryo-EM structure of the human uromodulin filament core reveals a unique assembly mechanism
Abstract
The glycoprotein uromodulin (UMOD) is the most abundant protein in human urine and forms filamentous homopolymers that encapsulate and aggregate uropathogens, promoting pathogen clearance by urine excretion. Despite its critical role in the innate immune response against urinary tract infections, the structural basis and mechanism of UMOD polymerization remained unknown. Here, we present the cryo-EM structure of the UMOD filament core at 3.5 Å resolution, comprised of the bipartite zona pellucida (ZP) module in a helical arrangement with a rise of ~65 Å and a twist of ~180°. The immunoglobulin-like ZPN and ZPC subdomains of each monomer are separated by a long linker that interacts with the preceding ZPC and following ZPN subdomains by β-sheet complementation. The unique filament architecture suggests an assembly mechanism in which subunit incorporation could be synchronized with proteolytic cleavage of the C-terminal pro-peptide that anchors assembly-incompetent UMOD precursors to the membrane.
Keywords: cryo-EM; glycoprotein; human; immunoglobulin-like fold; molecular biophysics; structural biology; uromodulin; zona pellucida module.
© 2020, Stanisich et al.
Conflict of interest statement
JS, DZ, PA, JX, AK, EO, OD, MP, DB, RG No competing interests declared
Figures

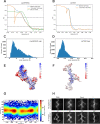
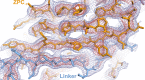

















Similar articles
-
Cryo-EM structure of native human uromodulin, a zona pellucida module polymer.EMBO J. 2020 Dec 15;39(24):e106807. doi: 10.15252/embj.2020106807. Epub 2020 Nov 16. EMBO J. 2020. PMID: 33196145 Free PMC article.
-
A structured interdomain linker directs self-polymerization of human uromodulin.Proc Natl Acad Sci U S A. 2016 Feb 9;113(6):1552-7. doi: 10.1073/pnas.1519803113. Epub 2016 Jan 25. Proc Natl Acad Sci U S A. 2016. PMID: 26811476 Free PMC article.
-
Structural basis of human uromodulin filament networks in uropathogen capture.Structure. 2025 Jul 3;33(7):1186-1192.e3. doi: 10.1016/j.str.2025.04.011. Epub 2025 May 8. Structure. 2025. PMID: 40345203
-
Structure of Zona Pellucida Module Proteins.Curr Top Dev Biol. 2018;130:413-442. doi: 10.1016/bs.ctdb.2018.02.007. Epub 2018 May 10. Curr Top Dev Biol. 2018. PMID: 29853186 Review.
-
Uromodulin biology and pathophysiology--an update.Kidney Blood Press Res. 2010;33(6):456-75. doi: 10.1159/000321013. Epub 2010 Nov 25. Kidney Blood Press Res. 2010. PMID: 21109754 Review.
Cited by
-
Microvilli regulate the release modes of alpha-tectorin to organize the domain-specific matrix architecture of the tectorial membrane.bioRxiv [Preprint]. 2024 Jan 5:2024.01.04.574255. doi: 10.1101/2024.01.04.574255. bioRxiv. 2024. PMID: 38260557 Free PMC article. Preprint.
-
UMOD and the architecture of kidney disease.Pflugers Arch. 2022 Aug;474(8):771-781. doi: 10.1007/s00424-022-02733-4. Epub 2022 Jul 26. Pflugers Arch. 2022. PMID: 35881244 Free PMC article. Review.
-
Identification of Six Novel Proteins Containing a ZP Module from Nemertean Species.Biomolecules. 2024 Dec 2;14(12):1545. doi: 10.3390/biom14121545. Biomolecules. 2024. PMID: 39766251 Free PMC article.
-
Donor-strand exchange drives assembly of the TasA scaffold in Bacillus subtilis biofilms.Nat Commun. 2022 Nov 18;13(1):7082. doi: 10.1038/s41467-022-34700-z. Nat Commun. 2022. PMID: 36400765 Free PMC article.
-
ZP2 cleavage blocks polyspermy by modulating the architecture of the egg coat.Cell. 2024 Mar 14;187(6):1440-1459.e24. doi: 10.1016/j.cell.2024.02.013. Cell. 2024. PMID: 38490181 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu Q, Jovine L, Rampoldi L. The serine protease hepsin mediates urinary secretion and polymerisation of zona pellucida domain protein uromodulin. eLife. 2015;4:e08887. doi: 10.7554/eLife.08887. - DOI - PMC - PubMed
-
- Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D Biological Crystallography. 2010;66:12–21. doi: 10.1107/S0907444909042073. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 310030B_176403/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/International
- 31003A_156304/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/International
- 31003A_179255/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/International
- 679209/H2020 European Research Council/International
- n/a/NOMIS Stiftung/International
- 310030_189044/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/International
- n/a/Swiss National Centre of Competence in Research Kidney Control of Homeostasis/International
- P2ZHP3_195181/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

