π-Extended Diaza[7]helicenes by Hybridization of Naphthalene Diimides and Hexa-peri-hexabenzocoronenes
- PMID: 32815577
- PMCID: PMC7898888
- DOI: 10.1002/chem.202003402
π-Extended Diaza[7]helicenes by Hybridization of Naphthalene Diimides and Hexa-peri-hexabenzocoronenes
Abstract
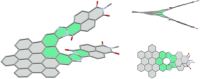
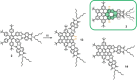
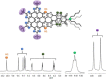
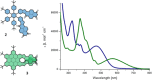
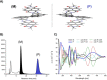
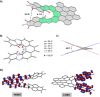
The synthesis of an unprecedented, π-extended hexabenzocorene (HBC)-based diaza[7]helicene is presented. The target compound was synthesized by an ortho-fusion of two naphthalene diimide (NDI) units to a HBC-skeleton. A combination of Diels-Alder and Scholl-type oxidation reactions involving a symmetric di-NDI-tolane precursor were crucial for the very selective formation of the helical superstructure via a hexaphenyl-benzene (HPB) derivative. The formation of the diaza[7]helicene moiety in the final Scholl oxidation is favoured, affording the symmetric π-extended helicene as the major product as a pair of enantiomers. The separation of the enantiomers was successfully accomplished by HPLC involving a chiral stationary phase. The absolute configuration of the enantiomers was assigned by comparison of circular dichroism spectra with quantum mechanical calculations.
Keywords: chirality; helicene; hexabenzocoronene; naphthalene; π-extension.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
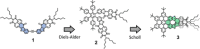



Similar articles
-
A Functional Hexaphenylbenzene Library Comprising of One, Three, and Six Peripheral Rylene-Diimide Substituents.Chemistry. 2021 Jan 21;27(5):1670-1679. doi: 10.1002/chem.202004273. Epub 2020 Dec 28. Chemistry. 2021. PMID: 33140885 Free PMC article.
-
Chiral Distorted Hexa-peri-hexabenzocoronenes Bearing a Nonagon-Embedded Carbohelicene.Angew Chem Int Ed Engl. 2021 Sep 27;60(40):22051-22056. doi: 10.1002/anie.202109310. Epub 2021 Aug 25. Angew Chem Int Ed Engl. 2021. PMID: 34329498 Free PMC article.
-
A Carbazole-Centered Expanded Helicene Stabilized with Hexabenzocoronene (HBC) Units.Angew Chem Int Ed Engl. 2025 Jan 27;64(5):e202417745. doi: 10.1002/anie.202417745. Epub 2024 Nov 25. Angew Chem Int Ed Engl. 2025. PMID: 39552120
-
Helicene Radicals: Molecules Bearing a Combination of Helical Chirality and Unpaired Electron Spin.Chempluschem. 2020 Sep;85(9):2093-2104. doi: 10.1002/cplu.202000452. Chempluschem. 2020. PMID: 32935935 Review.
-
Construction of hexabenzocoronene-based chiral nanographenes.Beilstein J Org Chem. 2023 May 30;19:736-751. doi: 10.3762/bjoc.19.54. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37284588 Free PMC article. Review.
Cited by
-
Synthesis and Chiroptical Activity of π-Expanded Electron-Rich Heterohelicenes Based on the 1,4-Dihydropyrrolo[3,2-b]pyrrole Core.Chemistry. 2025 Feb 25;31(12):e202404632. doi: 10.1002/chem.202404632. Epub 2025 Feb 5. Chemistry. 2025. PMID: 39838917 Free PMC article.
-
Helically and Linearly Fused Rylenediimide-Hexabenzocoronenes.Chemistry. 2021 Apr 12;27(21):6511-6521. doi: 10.1002/chem.202005235. Epub 2021 Mar 10. Chemistry. 2021. PMID: 33492668 Free PMC article.
-
Double helicene possessing B-N dative bonds built on 1,4-dihydropyrrolo[3,2-b]pyrrole core.Chem Sci. 2025 Apr 2;16(19):8338-8345. doi: 10.1039/d5sc00540j. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40213369 Free PMC article.
-
A Functional Hexaphenylbenzene Library Comprising of One, Three, and Six Peripheral Rylene-Diimide Substituents.Chemistry. 2021 Jan 21;27(5):1670-1679. doi: 10.1002/chem.202004273. Epub 2020 Dec 28. Chemistry. 2021. PMID: 33140885 Free PMC article.
-
Amplification of Dissymmetry Factors in π-Extended [7]- and [9]Helicenes.J Am Chem Soc. 2021 Mar 31;143(12):4661-4667. doi: 10.1021/jacs.0c13197. Epub 2021 Mar 18. J Am Chem Soc. 2021. PMID: 33735570 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

