π-Extended Diaza[7]helicenes by Hybridization of Naphthalene Diimides and Hexa-peri-hexabenzocoronenes
- PMID: 32815577
- PMCID: PMC7898888
- DOI: 10.1002/chem.202003402
π-Extended Diaza[7]helicenes by Hybridization of Naphthalene Diimides and Hexa-peri-hexabenzocoronenes
Abstract
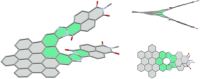
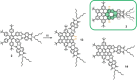
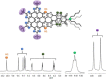
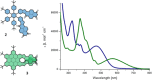
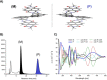
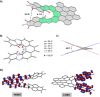
The synthesis of an unprecedented, π-extended hexabenzocorene (HBC)-based diaza[7]helicene is presented. The target compound was synthesized by an ortho-fusion of two naphthalene diimide (NDI) units to a HBC-skeleton. A combination of Diels-Alder and Scholl-type oxidation reactions involving a symmetric di-NDI-tolane precursor were crucial for the very selective formation of the helical superstructure via a hexaphenyl-benzene (HPB) derivative. The formation of the diaza[7]helicene moiety in the final Scholl oxidation is favoured, affording the symmetric π-extended helicene as the major product as a pair of enantiomers. The separation of the enantiomers was successfully accomplished by HPLC involving a chiral stationary phase. The absolute configuration of the enantiomers was assigned by comparison of circular dichroism spectra with quantum mechanical calculations.
Keywords: chirality; helicene; hexabenzocoronene; naphthalene; π-extension.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
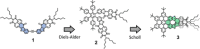



References
-
- None
-
- Cheung K. Y., Chan C. K., Liu Z., Miao Q., Angew. Chem. Int. Ed. 2017, 56, 9003–9007; - PubMed
- Angew. Chem. 2017, 129, 9131–9135;
-
- Fernández-García J. M., Evans P. J., Medina Rivero S., Fernández I., García-Fresnadillo D., Perles J., Casado J., Martín N., J. Am. Chem. Soc. 2018, 140, 17188–17196; - PubMed
-
- Majewski M. A., Stępień M., Angew. Chem. Int. Ed. 2019, 58, 86–116; - PubMed
- Angew. Chem. 2019, 131, 90–122;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

