Secukinumab efficacy in reducing the severity and the psychosocial impact of moderate-to-severe psoriasis as assessed by the Simplified Psoriasis Index: results from the IPSI-PSO study
- PMID: 32815591
- PMCID: PMC7984225
- DOI: 10.1111/jdv.16893
Secukinumab efficacy in reducing the severity and the psychosocial impact of moderate-to-severe psoriasis as assessed by the Simplified Psoriasis Index: results from the IPSI-PSO study
Abstract
Background: The utility of the Simplified Psoriasis Index (SPI), a recently developed multidomain tool for assessing psoriasis, was investigated in a study assessing response to secukinumab.
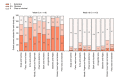
Methods: In an open-label, multicentre study involving 17 French centres, patients with moderate-to-severe plaque psoriasis received secukinumab 300 mg subcutaneously once weekly from baseline to W4, then every 4 weeks until W48. Dermatologist-scored SPI psoriasis severity (proSPI-s) was compared with Psoriasis Area and Severity Index (PASI). Patient self-assessed severity (saSPI-s) and psychosocial impact (SPI-p) were compared with PASI and Dermatology Life Quality Index (DLQI), respectively.
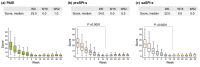
Results: We included 120 patients (69.2% male; mean age 45.9 years; mean duration of psoriasis 21.6 years). Mean baseline scores were as follows: proSPI-s 24.9, saSPI-s 23.5, PASI 23.1, SPI-p 8.2 and DLQI 13.6. Severity scores achieved by 16 weeks (proSPI-s 2.3, saSPI-s 2.2 and PASI 2.2) were maintained to W52. Reductions in mean psychosocial impact scores were maintained to W52 (SPI-p and DLQI, respectively, 2.1 and 1.5 at W16; 1.5 and 1.9 at W52).
Conclusions: Decrease of PASI scores in response to secukinumab was closely correlated with proSPI-s, supporting the latter's suitability for assessing response to therapy. Although the correlation between PASI and saSPI-s was slightly weaker, patients were able to complete a valid assessment of their psoriasis independently, and thus potentially remotely. With the added benefit of psychosocial impact assessment (SPI-p), SPI provides a valid tool enabling patients to assess their own psoriasis, remotely if necessary.
© 2020 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Lebwohl M, Swensen AR, Nyirady J et al. The Psoriasis Symptom Diary: development and content validity of a novel patient‐reported outcome instrument. Int J Dermatol 2014; 53: 714–722. - PubMed
-
- Langley RG, Elewski BE, Lebwohl M et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. - PubMed
-
- Blauvelt A, Prinz JC, Gottlieb AB et al. Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172: 484–493. - PubMed
-
- Paul C, Lacour J‐P, Tedremets L et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29: 1082–1090. - PubMed
-
- Thaçi D, Blauvelt A, Reich K et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73: 400–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

