Sensitive and Specific Detection of SARS-CoV-2 Antibodies Using a High-Throughput, Fully Automated Liquid-Handling Robotic System
- PMID: 32815769
- PMCID: PMC7441286
- DOI: 10.1177/2472630320950663
Sensitive and Specific Detection of SARS-CoV-2 Antibodies Using a High-Throughput, Fully Automated Liquid-Handling Robotic System
Abstract
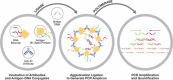
As of July 22, 2020, more than 14.7 million infections of SARS-CoV-2, the virus responsible for Coronavirus Disease 2019 (COVID-19), have been confirmed globally. Serological assays are essential for community screening, assessing infection prevalence, aiding identification of infected patients, and enacting appropriate treatment and quarantine protocols in the battle against this rapidly expanding pandemic. Antibody detection by agglutination-PCR (ADAP) is a pure solution phase immunoassay that generates a PCR amplifiable signal when patient antibodies agglutinate DNA-barcoded antigen probes into a dense immune complex. Here, we present an ultrasensitive and high-throughput automated liquid biopsy assay based on the Hamilton Microlab ADAP STAR automated liquid-handling platform, which was developed and validated for the qualitative detection of total antibodies against spike protein 1 (S1) of SARS-CoV-2 that uses as little as 4 µL of serum. To assess the clinical performance of the ADAP assay, 57 PCR-confirmed COVID-19 patients and 223 control patients were tested. The assay showed a sensitivity of 98% (56/57) and a specificity of 99.55% (222/223). Notably, the SARS-CoV-2-negative control patients included individuals with other common coronaviral infections, such as CoV-NL63 and CoV-HKU, which did not cross-react. In addition to high performance, the hands-free automated workstation enabled high-throughput sample processing to reduce screening workload while helping to minimize analyst contact with biohazardous samples. Therefore, the ADAP STAR liquid-handling workstation can be used as a valuable tool to address the COVID-19 global pandemic.
Keywords: PCR; agglutination; antibody; anti–spike protein; liquid handling.
Figures


References
-
- Long Q.X., Liu B.Z., Deng H.J., et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020;26:845–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

