Pharmacogenetic interactions of rifapentine plus isoniazid with efavirenz or nevirapine
- PMID: 32815870
- PMCID: PMC7655626
- DOI: 10.1097/FPC.0000000000000417
Pharmacogenetic interactions of rifapentine plus isoniazid with efavirenz or nevirapine
Abstract
Objectives: The effect of rifapentine plus isoniazid on efavirenz pharmacokinetics was characterized in AIDS Clinical Trials Group protocol A5279 (NCT01404312). The present analyses characterize pharmacogenetic interactions between these drugs, and with nevirapine.
Methods: A subset of HIV-positive individuals receiving efavirenz- or nevirapine-containing antiretroviral therapy in A5279 underwent pharmacokinetic evaluations at baseline, and again weeks 2 and 4 after initiating daily rifapentine plus isoniazid. Associations with polymorphisms relevant to efavirenz, nevirapine, isoniazid, and rifapentine pharmacokinetics were assessed.
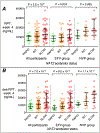
Results: Of 128 participants, 101 were evaluable for associations with rifapentine and its active 25-desacetyl metabolite, 87 with efavirenz, and 38 with nevirapine. In multivariable analyses, NAT2 slow acetylators had greater week 4 plasma concentrations of rifapentine (P = 2.6 × 10) and 25-desacetyl rifapentine (P = 7.0 × 10) among all participants, and in efavirenz and nevirapine subgroups. NAT2 slow acetylators also had greater plasma efavirenz and nevirapine concentration increases from baseline to week 4, and greater decreases from baseline in clearance. CYP2B6 poor metabolizers had greater efavirenz concentrations at all weeks and greater nevirapine concentrations at baseline. None of 47 additional polymorphisms in 11 genes were significantly associated with pharmacokinetics.
Conclusions: Among HIV-positive individuals receiving efavirenz or nevirapine, and who then initiated rifapentine plus isoniazid in A5279, NAT2 slow acetylators had greater rifapentine and 25-desacetyl rifapentine concentrations, and greater increases from baseline in plasma efavirenz and nevirapine concentrations. These associations are likely mediated by greater isoniazid exposure in NAT2 slow acetylators.
Conflict of interest statement
Declaration of Interests:
Susan Swindells: reports research grants to her institution from ViiV Healthcare.
Richard E. Chaisson: Has consulted for Sanofi. His spouse is Merck stockholder.
Other authors have nothing to report
Figures


References
-
- World Health Organization. Tuberculosis (TB). 2019. Available at: https://www.who.int/tb/areas-of-work/tb-hiv/en/. Accessed January 31, 2019.
-
- Horsburgh CR Jr., Goldberg S, Bethel J, Chen S, Colson PW, Hirsch-Moverman Y, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–9. - PubMed
-
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. The New England journal of medicine. 2011;365(23):2155–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069438/AI/NIAID NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- K23 AI134307/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- R01 AI120790/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States

