Low-dose radiotherapy for COVID-19 pneumonia treatment: case report, procedure, and literature review
- PMID: 32816059
- PMCID: PMC7439803
- DOI: 10.1007/s00066-020-01675-z
Low-dose radiotherapy for COVID-19 pneumonia treatment: case report, procedure, and literature review
Abstract
Background: The COVID-19 pandemic outbreak has set the emergency services in developing countries on major alert, as the installed response capacities are easily overwhelmed by the constantly increasing high demand. The deficit of intensive care unit beds and ventilators in countries like Peru is forcing practitioners to seek preventive or early interventional strategies to prevent saturating these chronically neglected facilities.
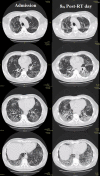
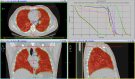
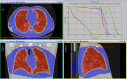
Case presentation: A 64-year-old patient is reported after presenting with COVID-19 pneumonia and rapidly progressing to deteriorated ventilatory function. Compassionate treatment with a single 1‑Gy dose to the bilateral whole-lung volume was administered, with gradual daily improvement of ventilatory function and decrease in serum inflammatory markers and oxygen support needs, including intubation. No treatment-related toxicity developed. Procedures of transport, disinfection, and treatment planning and delivery are described.
Conclusion: Whole-lung low-dose radiotherapy seems to be a promising approach for avoiding or delaying invasive respiratory support. Delivered low doses are far from meeting toxicity ranges. On-going prospective trials will elucidate the effectiveness of this approach.
Keywords: COVID-19; Cytokine storm; Intensive care; Low-dose radiotherapy; Viral pneumonia.
Conflict of interest statement
R. Del Castillo, D. Martinez, G.J. Sarria, L. Pinillos, B. Garcia, L. Castillo, and A. Carhuactocto declare that they have no competing interests. F.A. Giordano reports personal fees from Implacit GmbH, non-financial support from Oncare GmbH, grants and personal fees from NOXXON Pharma AG, grants and personal fees from CARL ZEISS MEDITEC AG, personal fees from Bristol-Myers Squibb, personal fees from Roche Pharma AG, personal fees from MSD Sharp and Dohme GmbH, personal fees from AstraZeneca GmbH, outside the submitted work. In addition, F.A. Giordano has a patent (US 62/435405) pending, not related to the submitted work. G.R. Sarria reports grants and personal fees from Carl Zeiss Meditec AG, not related to the submitted work.
Figures
References
-
- Available from: https://rpp.pe/politica/gobierno/coronavius-comando-covid-quedan-185-cam.... Accessed 29 May 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

