Tissue-specific Nrf2 signaling protects against methylmercury toxicity in Drosophila neuromuscular development
- PMID: 32816092
- PMCID: PMC7657992
- DOI: 10.1007/s00204-020-02879-z
Tissue-specific Nrf2 signaling protects against methylmercury toxicity in Drosophila neuromuscular development
Abstract
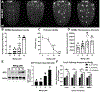
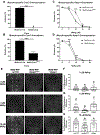
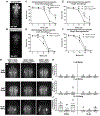
Methylmercury (MeHg) can elicit cognitive and motor deficits due to its developmental neuro- and myotoxic properties. While previous work has demonstrated that Nrf2 antioxidant signaling protects from MeHg toxicity, in vivo tissue-specific studies are lacking. In Drosophila, MeHg exposure shows greatest developmental toxicity in the pupal stage resulting in failed eclosion (emergence of adults) and an accompanying 'myosphere' phenotype in indirect flight muscles (IFMs). To delineate tissue-specific contributions to MeHg-induced motor deficits, we investigated the potential of Nrf2 signaling in either muscles or neurons to moderate MeHg toxicity. Larva were exposed to various concentrations of MeHg (0-20 µM in food) in combination with genetic modulation of the Nrf2 homolog cap-n-collar C (CncC), or its negative regulator Keap1. Eclosion behavior was evaluated in parallel with the morphology of two muscle groups, the thoracic IFMs and the abdominal dorsal internal oblique muscles (DIOMs). CncC signaling activity was reported with an antioxidant response element construct (ARE-GFP). We observed that DIOMs are distinguished by elevated endogenous ARE-GFP expression, which is only transiently seen in the IFMs. Dose-dependent MeHg reductions in eclosion behavior parallel formation of myospheres in the DIOMs and IFMs, while also increasing ARE-GFP expression in the DIOMs. Modulating CncC signaling via muscle-specific Keap1 knockdown and upregulation gives a rescue and exacerbation, respectively, of MeHg effects on eclosion and myospheres. Interestingly, muscle-specific CncC upregulation and knockdown both induce lethality. In contrast, neuron-specific upregulation of CncC, as well as Keap1 knockdown, rescued MeHg effects on eclosion and myospheres. Our findings indicate that enhanced CncC signaling localized to either muscles or neurons is sufficient to rescue muscle development and neuromuscular function from a MeHg insult. Additionally, there may be distinct roles for CncC signaling in myo-morphogenesis.
Keywords: Drosophila; Methylmercury; Myotoxicity; Neuromuscular development; Nrf2 signaling.
Conflict of interest statement
Figures





Similar articles
-
Methylmercury myotoxicity targets formation of the myotendinous junction.Toxicology. 2020 Oct;443:152561. doi: 10.1016/j.tox.2020.152561. Epub 2020 Aug 13. Toxicology. 2020. PMID: 32800841 Free PMC article.
-
Genome-wide association analysis of tolerance to methylmercury toxicity in Drosophila implicates myogenic and neuromuscular developmental pathways.PLoS One. 2014 Oct 31;9(10):e110375. doi: 10.1371/journal.pone.0110375. eCollection 2014. PLoS One. 2014. PMID: 25360876 Free PMC article.
-
The Notch target E(spl)mδ is a muscle-specific gene involved in methylmercury toxicity in motor neuron development.Neurotoxicol Teratol. 2014 May-Jun;43:11-8. doi: 10.1016/j.ntt.2014.03.001. Epub 2014 Mar 13. Neurotoxicol Teratol. 2014. PMID: 24632433 Free PMC article.
-
The role of the Keap1/Nrf2 pathway in the cellular response to methylmercury.Oxid Med Cell Longev. 2013;2013:848279. doi: 10.1155/2013/848279. Epub 2013 Jun 26. Oxid Med Cell Longev. 2013. PMID: 23878621 Free PMC article. Review.
-
Review: myogenic and muscle toxicity targets of environmental methylmercury exposure.Arch Toxicol. 2024 Jun;98(6):1645-1658. doi: 10.1007/s00204-024-03724-3. Epub 2024 Mar 28. Arch Toxicol. 2024. PMID: 38546836 Free PMC article. Review.
Cited by
-
Geraniol attenuates oxidative stress and neuroinflammation-mediated cognitive impairment in D galactose-induced mouse aging model.Aging (Albany NY). 2024 Mar 20;16(6):5000-5026. doi: 10.18632/aging.205677. Epub 2024 Mar 20. Aging (Albany NY). 2024. PMID: 38517361 Free PMC article.
-
Transcriptomic analysis identifies muscle-specific mitochondrial and vesicular transport genes as methylmercury toxicity targets in a Drosophila model of congenital Minamata disease.Toxicol Sci. 2025 May 1;205(1):106-123. doi: 10.1093/toxsci/kfaf018. Toxicol Sci. 2025. PMID: 39951334
-
KEAP1 polymorphisms and neurodevelopmental outcomes in children with exposure to prenatal MeHg from the Seychelles Child Development Study Nutrition Cohort 2.Neurotoxicology. 2023 Dec;99:177-183. doi: 10.1016/j.neuro.2023.10.008. Epub 2023 Oct 17. Neurotoxicology. 2023. PMID: 37858899 Free PMC article.
-
Gene expression variation underlying tissue-specific responses to copper stress in Drosophila melanogaster.G3 (Bethesda). 2024 Mar 6;14(3):jkae015. doi: 10.1093/g3journal/jkae015. G3 (Bethesda). 2024. PMID: 38262701 Free PMC article.
-
Gene expression variation underlying tissue-specific responses to copper stress in Drosophila melanogaster.bioRxiv [Preprint]. 2023 Jul 18:2023.07.12.548746. doi: 10.1101/2023.07.12.548746. bioRxiv. 2023. Update in: G3 (Bethesda). 2024 Mar 6;14(3):jkae015. doi: 10.1093/g3journal/jkae015. PMID: 37503205 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

