Natural Diversity in Stomatal Features of Cultivated and Wild Oryza Species
- PMID: 32816163
- PMCID: PMC7441136
- DOI: 10.1186/s12284-020-00417-0
Natural Diversity in Stomatal Features of Cultivated and Wild Oryza Species
Abstract
Background: Stomata in rice control a number of physiological processes by regulating gas and water exchange between the atmosphere and plant tissues. The impact of the structural diversity of these micropores on its conductance level is an important area to explore before introducing stomatal traits into any breeding program in order to increase photosynthesis and crop yield. Therefore, an intensive measurement of structural components of stomatal complex (SC) of twenty three Oryza species spanning the primary, secondary and tertiary gene pools of rice has been conducted.
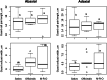
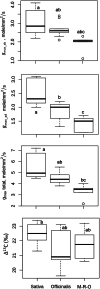
Results: Extensive diversity was found in stomatal number and size in different Oryza species and Oryza complexes. Interestingly, the dynamics of stomatal traits in Oryza family varies differently within different Oryza genetic complexes. Example, the Sativa complex exhibits the greatest diversity in stomatal number, while the Officinalis complex is more diverse for its stomatal size. Combining the structural information with the Oryza phylogeny revealed that speciation has tended towards increasing stomatal density rather than stomatal size in rice family. Thus, the most recent species (i.e. the domesticated rice) eventually has developed smaller yet numerous stomata. Along with this, speciation has also resulted in a steady increase in stomatal conductance (anatomical, gmax) in different Oryza species. These two results unambiguously prove that increasing stomatal number (which results in stomatal size reduction) has increased the stomatal conductance in rice. Correlations of structural traits with the anatomical conductance, leaf carbon isotope discrimination (∆13C) and major leaf morphological and anatomical traits provide strong supports to untangle the ever mysterious dependencies of these traits in rice. The result displayed an expected negative correlation in the number and size of stomata; and positive correlations among the stomatal length, width and area with guard cell length, width on both abaxial and adaxial leaf surfaces. In addition, gmax is found to be positively correlated with stomatal number and guard cell length. The ∆13C values of rice species showed a positive correlation with stomatal number, which suggest an increased water loss with increased stomatal number. Interestingly, in contrast, the ∆13C consistently shows a negative relationship with stomatal and guard cell size, which suggests that the water loss is less when the stomata are larger. Therefore, we hypothesize that increasing stomatal size, instead of numbers, is a better approach for breeding programs in order to minimize the water loss through stomata in rice.
Conclusion: Current paper generates useful data on stomatal profile of wild rice that is hitherto unknown for the rice science community. It has been proved here that the speciation has resulted in an increased stomatal number accompanied by size reduction during Oryza's evolutionary course; this has resulted in an increased gmax but reduced water use efficiency. Although may not be the sole driver of water use efficiency in rice, our data suggests that stomata are a potential target for modifying the currently low water use efficiency in domesticated rice. It is proposed that Oryza barthii can be used in traditional breeding programs in enhancing the stomatal size of elite rice cultivars.
Keywords: Maximum stomatal conductance (anatomical); Oryza; Stomatal diversity; Wild rice; gmax.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Bergmann DC, Sack FD. Stomatal development. Annu Rev Plant Biol. 2007;58:163–181. - PubMed
-
- Brodribb T, Jordan GJ, Carpenter RJ. Unified changes in cell size permit coordinated leaf evolution. New Phytol. 2013;199:559–570. - PubMed
-
- Caine RS, Yin X, Sloan J, Harrison EL, Mohammed U, Fulton T, Biswal AK, Dionora J, Chater CC, Coe RA, Bandyopadhyay A, Murchie EH, Swarup R, Quick WP, Gray JE. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018;221:371–384. - PMC - PubMed
-
- Chen WF, Xu ZJ, Zhang LB, Yang SR. Comparative studies on stomatal density and its relations to gas diffusion resistance and net photosynthetic rate in rice leaf. Chinese J Rice Sci. 1990;4:63–168.
LinkOut - more resources
Full Text Sources

