Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial
- PMID: 32816842
- PMCID: PMC7500441
- DOI: 10.1136/bmj.m2836
Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial
Abstract
Objective: To determine whether risk adapted intraoperative radiotherapy, delivered as a single dose during lumpectomy, can effectively replace postoperative whole breast external beam radiotherapy for early breast cancer.
Design: Prospective, open label, randomised controlled clinical trial.
Setting: 32 centres in 10 countries in the United Kingdom, Europe, Australia, the United States, and Canada.
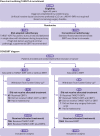
Participants: 2298 women aged 45 years and older with invasive ductal carcinoma up to 3.5 cm in size, cN0-N1, eligible for breast conservation and randomised before lumpectomy (1:1 ratio, blocks stratified by centre) to either risk adapted targeted intraoperative radiotherapy (TARGIT-IORT) or external beam radiotherapy (EBRT).
Interventions: Random allocation was to the EBRT arm, which consisted of a standard daily fractionated course (three to six weeks) of whole breast radiotherapy, or the TARGIT-IORT arm. TARGIT-IORT was given immediately after lumpectomy under the same anaesthetic and was the only radiotherapy for most patients (around 80%). TARGIT-IORT was supplemented by EBRT when postoperative histopathology found unsuspected higher risk factors (around 20% of patients).
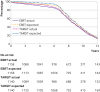
Main outcome measures: Non-inferiority with a margin of 2.5% for the absolute difference between the five year local recurrence rates of the two arms, and long term survival outcomes.
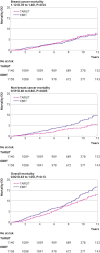
Results: Between 24 March 2000 and 25 June 2012, 1140 patients were randomised to TARGIT-IORT and 1158 to EBRT. TARGIT-IORT was non-inferior to EBRT: the local recurrence risk at five year complete follow-up was 2.11% for TARGIT-IORT compared with 0.95% for EBRT (difference 1.16%, 90% confidence interval 0.32 to 1.99). In the first five years, 13 additional local recurrences were reported (24/1140 v 11/1158) but 14 fewer deaths (42/1140 v 56/1158) for TARGIT-IORT compared with EBRT. With long term follow-up (median 8.6 years, maximum 18.90 years, interquartile range 7.0-10.6) no statistically significant difference was found for local recurrence-free survival (hazard ratio 1.13, 95% confidence interval 0.91 to 1.41, P=0.28), mastectomy-free survival (0.96, 0.78 to 1.19, P=0.74), distant disease-free survival (0.88, 0.69 to 1.12, P=0.30), overall survival (0.82, 0.63 to 1.05, P=0.13), and breast cancer mortality (1.12, 0.78 to 1.60, P=0.54). Mortality from other causes was significantly lower (0.59, 0.40 to 0.86, P=0.005).
Conclusion: For patients with early breast cancer who met our trial selection criteria, risk adapted immediate single dose TARGIT-IORT during lumpectomy was an effective alternative to EBRT, with comparable long term efficacy for cancer control and lower non-breast cancer mortality. TARGIT-IORT should be discussed with eligible patients when breast conserving surgery is planned.
Trial registration: ISRCTN34086741, NCT00983684.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from University College London Hospitals (UCLH)/UCL Comprehensive Biomedical Research Centre, UCLH Charities, National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, Ninewells Cancer Campaign, National Health and Medical Research Council, German Federal Ministry of Education and Research (BMBF), and Cancer Research Campaign (now Cancer Research UK) for the submitted work; JSV has received a research grant from Photoelectron Corp (1996-99) and from Carl Zeiss for supporting data management at the University of Dundee (Dundee, UK, 2004-2008), and has received honorariums. JSV, JST, NRW, IP, CBG, and NR receive funding from HTA, NIHR, Department of Health and Social Care for some activities related to the TARGIT trials. MBa was briefly on the scientific advisory board of Carl Zeiss and was paid consultancy fees before 2010. FW has received a research grant from Carl Zeiss for supporting radiobiological research. Carl Zeiss sponsors some of the travel and accommodation for meetings of the international steering committee and data monitoring committee and when necessary for conferences where a presentation about targeted intraoperative radiotherapy is being made for all authors apart from WE who declares that he has no conflicts of interest. All other authors declare that they have no conflicts of interest.
Figures


Comment in
-
Intraoperative radiotherapy for early breast cancer - insufficient evidence to change practice.Nat Rev Clin Oncol. 2020 Dec;17(12):723-724. doi: 10.1038/s41571-020-00444-2. Nat Rev Clin Oncol. 2020. PMID: 33087894 Free PMC article.
-
Reply to 'Intraoperative radiotherapy for breast cancer: powerful evidence to change practice'.Nat Rev Clin Oncol. 2021 Mar;18(3):188-189. doi: 10.1038/s41571-021-00472-6. Nat Rev Clin Oncol. 2021. PMID: 33495551 No abstract available.
-
Intraoperative radiotherapy for breast cancer: powerful evidence to change practice.Nat Rev Clin Oncol. 2021 Mar;18(3):187-188. doi: 10.1038/s41571-021-00471-7. Nat Rev Clin Oncol. 2021. PMID: 33495552 Free PMC article. No abstract available.
-
Single-dose intraoperative radiotherapy during lumpectomy for breast cancer: an innovative patient-centred treatment.Br J Cancer. 2021 Apr;124(9):1469-1474. doi: 10.1038/s41416-020-01233-5. Epub 2021 Feb 2. Br J Cancer. 2021. PMID: 33531693 Free PMC article.
References
-
- Vaidya JS, Baum M, Tobias JS, et al. Targeted Intraoperative Radiothearpy (TARGIT)-trial protocol. Lancet 1999. https://www.thelancet.com/protocol-reviews/99PRT-47.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
