Race-Dependent Differences in Risk, Genomics, and Epstein-Barr Virus Exposure in Monoclonal Gammopathies: Results of SWOG S0120
- PMID: 32816893
- PMCID: PMC7679008
- DOI: 10.1158/1078-0432.CCR-20-2119
Race-Dependent Differences in Risk, Genomics, and Epstein-Barr Virus Exposure in Monoclonal Gammopathies: Results of SWOG S0120
Abstract
Purpose: Risk of multiple myeloma is increased in African American (AA) populations compared with European American (EA) cohorts. Current estimates of risk of progression of monoclonal gammopathy of undetermined significance (MGUS) are based largely on studies in EA cohorts. Prospective analyses of this risk in AA cohorts are lacking.
Patients and methods: Between 2003 and 2011, 331 eligible patients with IgG/A monoclonal gammopathy were enrolled in a prospective observational trial (SWOG S0120).
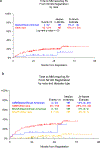
Results: Of 331 eligible patients, 57 (17%) were of AA descent. The risk of transformation to clinical malignancy in AA patients was significantly lower than in non-AA cohort (2-year risk 5% vs. 15%; 5-year risk 13% vs. 24%; log-rank P = 0.047). Differences in risk were evident for both MGUS and asymptomatic multiple myeloma. Gene expression profile (GEP) of CD138-purified plasma cells revealed that all molecular multiple myeloma subsets can be identified in both cohorts. However, the proportion of patients with high-risk GEP risk score (GEP-70 gene risk > -0.26) was lower in the AA cohort (0% vs. 33%, P = 0.01). AA cohorts also have higher levels of antibodies against Epstein-Barr nuclear antigen-1 (EBNA-1; P < 0.001).
Conclusions: These data provide the first prospective evidence that multiple myeloma precursor states in AA patients may have lower risk of disease compared with non-AA counterparts with lower incidence of high-risk GEP and increased EBV seropositivity. Race-dependent differences in biology and clinical risk of gammopathy may impact optimal management of these patients.
Trial registration: ClinicalTrials.gov NCT00900263.
©2020 American Association for Cancer Research.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA068183/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- R35 CA197603/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

