A Compass To Boost Navigation: Cell Biology of Bacterial Magnetotaxis
- PMID: 32817094
- PMCID: PMC7549360
- DOI: 10.1128/JB.00398-20
A Compass To Boost Navigation: Cell Biology of Bacterial Magnetotaxis
Abstract
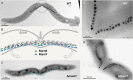
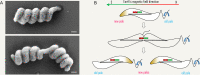
Magnetotactic bacteria are aquatic or sediment-dwelling microorganisms able to take advantage of the Earth's magnetic field for directed motility. The source of this amazing trait is magnetosomes, unique organelles used to synthesize single nanometer-sized crystals of magnetic iron minerals that are queued up to build an intracellular compass. Most of these microorganisms cannot be cultivated under controlled conditions, much less genetically engineered, with only few exceptions. However, two of the genetically amenable Magnetospirillum species have emerged as tractable model organisms to study magnetosome formation and magnetotaxis. Recently, much has been revealed about the process of magnetosome biogenesis and dedicated structures for magnetosome dynamics and positioning, which suggest an unexpected cellular intricacy of these organisms. In this minireview, we summarize new insights and place the molecular mechanisms of magnetosome formation in the context of the complex cell biology of Magnetospirillum spp. First, we provide an overview on magnetosome vesicle synthesis and magnetite biomineralization, followed by a discussion of the perceptions of dynamic organelle positioning and its biological implications, which highlight that magnetotactic bacteria have evolved sophisticated mechanisms to construct, incorporate, and inherit a unique navigational device. Finally, we discuss the impact of magnetotaxis on motility and its interconnection with chemotaxis, showing that magnetotactic bacteria are outstandingly adapted to lifestyle and habitat.
Keywords: cell shape; cytoskeleton; flagella; magnetoskeleton; magnetosome; magnetospirillum; magnetotaxis.
Copyright © 2020 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

