Broad Anti-coronavirus Activity of Food and Drug Administration-Approved Drugs against SARS-CoV-2 In Vitro and SARS-CoV In Vivo
- PMID: 32817221
- PMCID: PMC7565640
- DOI: 10.1128/JVI.01218-20
Broad Anti-coronavirus Activity of Food and Drug Administration-Approved Drugs against SARS-CoV-2 In Vitro and SARS-CoV In Vivo
Abstract
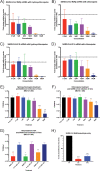
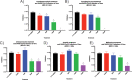
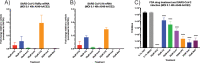
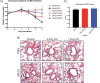
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China at the end of 2019 and has rapidly caused a pandemic, with over 20 million recorded COVID-19 cases in August 2020 (https://covid19.who.int/). There are no FDA-approved antivirals or vaccines for any coronavirus, including SARS-CoV-2. Current treatments for COVID-19 are limited to supportive therapies and off-label use of FDA-approved drugs. Rapid development and human testing of potential antivirals is urgently needed. Numerous drugs are already approved for human use, and subsequently, there is a good understanding of their safety profiles and potential side effects, making them easier to fast-track to clinical studies in COVID-19 patients. Here, we present data on the antiviral activity of 20 FDA-approved drugs against SARS-CoV-2 that also inhibit SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). We found that 17 of these inhibit SARS-CoV-2 at non-cytotoxic concentrations. We directly followed up seven of these to demonstrate that all are capable of inhibiting infectious SARS-CoV-2 production. Moreover, we evaluated two of these, chloroquine and chlorpromazine, in vivo using a mouse-adapted SARS-CoV model and found that both drugs protect mice from clinical disease.IMPORTANCE There are no FDA-approved antivirals for any coronavirus, including SARS-CoV-2. Numerous drugs are already approved for human use that may have antiviral activity and therefore could potentially be rapidly repurposed as antivirals. Here, we present data assessing the antiviral activity of 20 FDA-approved drugs against SARS-CoV-2 that also inhibit SARS-CoV and MERS-CoV in vitro We found that 17 of these inhibit SARS-CoV-2, suggesting that they may have pan-anti-coronaviral activity. We directly followed up seven of these and found that they all inhibit infectious-SARS-CoV-2 production. Moreover, we evaluated chloroquine and chlorpromazine in vivo using mouse-adapted SARS-CoV. We found that neither drug inhibited viral replication in the lungs, but both protected against clinical disease.
Keywords: FDA-approved drugs; SARS-CoV-2; antiviral therapeutics; coronavirus; drug repurposing; nCoV-2019; pandemic.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, Green CE, Iyer LV, Dilks HH, Davey RA, Kolokoltsov AA, Carrion R, Patterson JL, Bavari S, Panchal RG, Warren TK, Wells JB, Moos WH, Burke RLL, Tanga MJ. 2013. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One 8:e60579. doi: 10.1371/journal.pone.0060579. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

