Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2
- PMID: 32817233
- PMCID: PMC7587122
- DOI: 10.1128/JCM.01824-20
Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2
Abstract
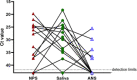
We prospectively compared health care worker-collected nasopharyngeal swabs (NPS) to self-collected anterior nasal swabs (ANS) and straight saliva for the diagnosis of coronavirus disease 2019 (COVID-19) in 354 patients. The percent positive agreement between NPS and ANS or saliva was 86.3% (95% confidence interval [CI], 76.7 to 92.9%) and 93.8% (95% CI, 86.0 to 97.9%), respectively. The percent negative agreement was 99.6% (95% CI, 98.0 to 100.0%) for NPS versus ANS and 97.8% (95% CI, 95.3 to 99.2%) for NPS versus saliva. More cases were detected by the use of NPS (n = 80) and saliva (n = 81) than by the use of ANS (n = 70), but no single specimen type detected all severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.
Keywords: SARS-Cov-2; alternative specimen; anterior nasal swab; nasopharyngeal swab; saliva.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Centers for Disease Control and Prevention. 2020. Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. CDC, Atlanta, GA: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 14 July 2020.
-
- To KK, Tsang OT, Chik-Yan Yip C, Chan KH, Wu TC, Chan JMC, Leung WS, Chik TS, Choi CY, Kandamby DH, Lung DC, Tam AR, Poon RW, Fung AY, Hung IF, Cheng VC, Chan JF, Yuen KY. 2020. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 71:841–843. doi:10.1093/cid/ciaa149. - DOI - PMC - PubMed
-
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, Lau DP, Choi CY, Chen LL, Chan WM, Chan KH, Ip JD, Ng AC, Poon RW, Luo CT, Cheng VC, Chan JF, Hung IF, Chen Z, Chen H, Yuen KY. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi:10.1016/S1473-3099(20)30196-1. - DOI - PMC - PubMed
-
- Altamirano J, Govindarajan P, Blomkalns AL, Kushner LE, Stevens BA, Pinsky BA, Maldonado Y. 2020. Assessment of sensitivity and specificity of patient-collected lower nasal specimens for sudden acute respiratory syndrome coronavirus 2 testing. JAMA Netw Open 3:e2012005. doi:10.1001/jamanetworkopen.2020.12005. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

